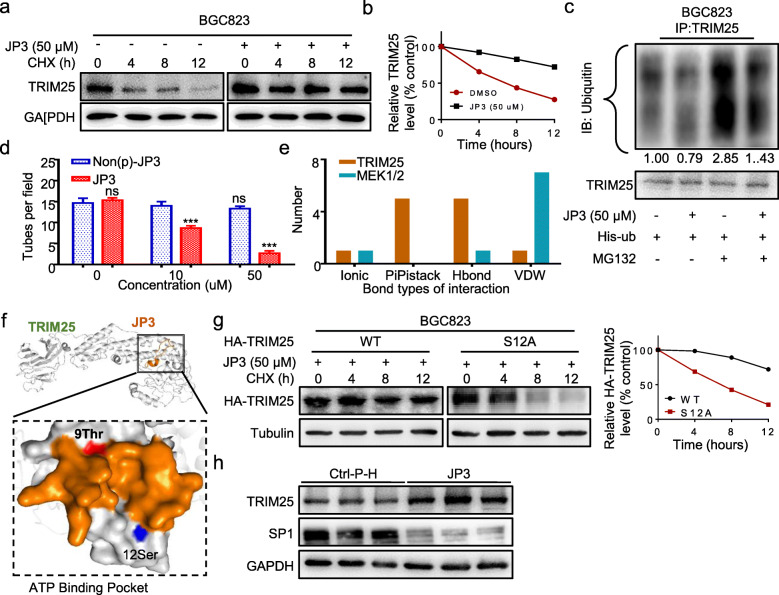
Fig. 5.
JP3 promotes the enhancement of phosphorylation at S12 and stabilization of TRIM25 in vitro. a BGC823 cells were treated with JP3 (0 or 50 μM), and then with CHX and harvested at the indicated time points for Western blotting. b The relative intensities of the TRIM25 protein bands were analyzed by densitometry after normalization to that of GAPDH. c BGC823 cells were transfected with His-Ub for 48 h, and then treated with JP3 (0 or 50 μM) for another 24 h, followed by pre-treatment with MG132 (10 μM) for 6 h. Ubiquitinated TRIM25 was determined by IP with anti-TRIM25, and the indicated protein levels were determined by Western blotting. d Tube formation assay was performed. The tube number was analyzed (means ± SEM, n = 3). ns: no significance, ***P < 0.001. e The main interaction types between amino acids between JP3 and TRIM25 or JP3 and MEK1/2. f A low-energy complex structure of JP3 and TRIM25 was predicted by a combination of Discovery Studio 3.0 and Rosetta software. g BGC823 cells were transfected with HA-TRIM25 wide type or S12A mutant for 36 h, followed by treatment of JP3 (50 μM) for another 12 h, treated with CHX and harvested at the indicated time points for Western blotting. The relative intensities of the HA-TRIM25 protein bands were analyzed by densitometry after normalization to that of Tubulin. h The expression levels of TRIM25 and SP1 protein were analyzed using Western blotting in tumor tissues from nude mice bearing GC