Figure 4 |. Structure-based mutational analysis of the AaBioW active site.
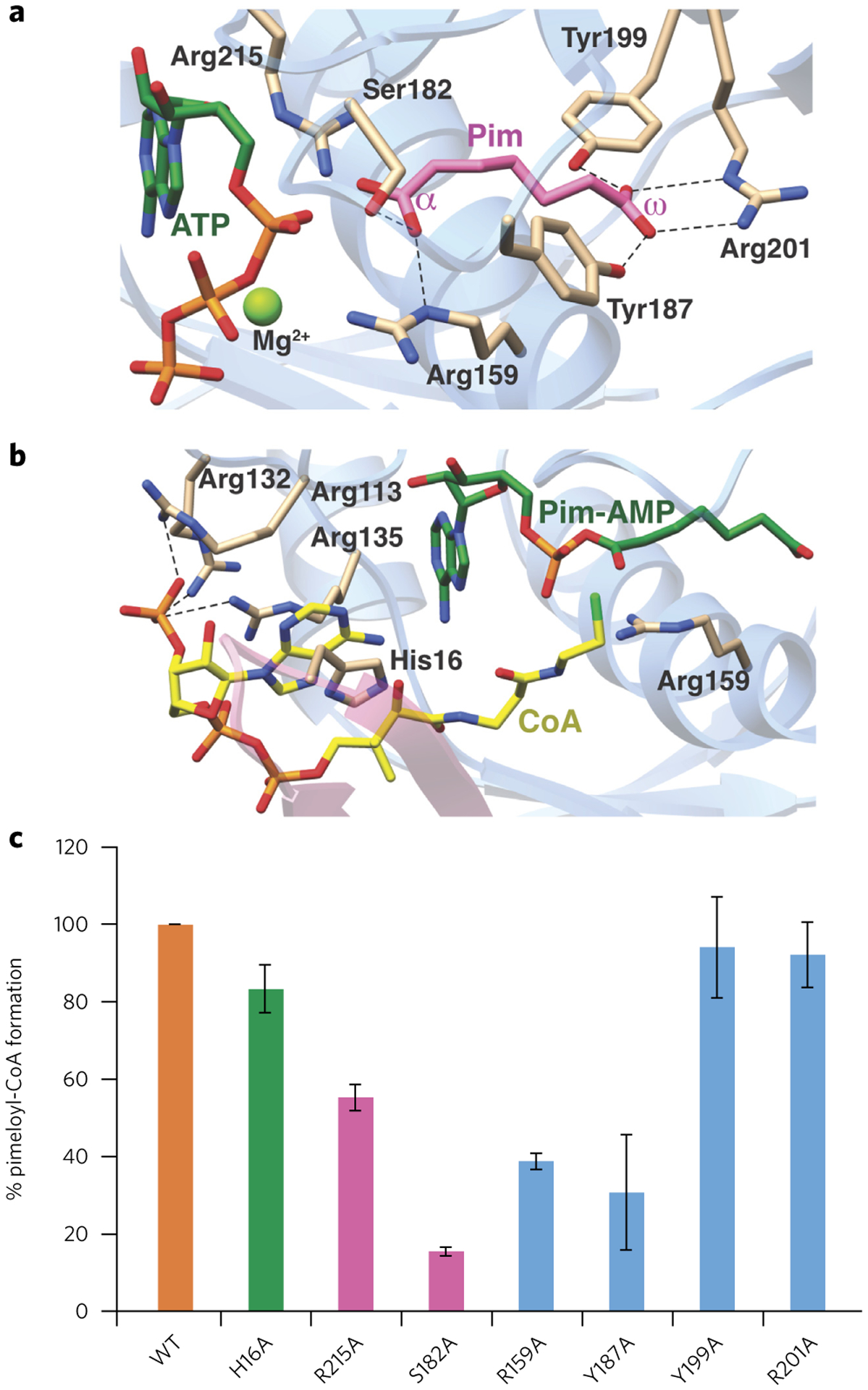
(a,b) Model of the AaBioW active site structure during the adenylation (a) and thioester (b) formation steps, generated by superimposing the crystal structures of relevant ligand-bond complexes. Green sticks, nucleotide; purple, pimelate (Pim); yellow, CoA. Active site residues that may play a role in catalysis are shown as tan sticks. (c) Biochemical activities of site-specific variants of active site residues in AaBioW are identified in a and b. The efficiency of each variant is measured as the amount of pimeloyl-CoA formed relative to the wild-type enzyme. Experiments in c were conducted in triplicate. Error bars represent mean ± s.d.
