Figure 5 |. Proofreading activity of the wild-type and R159A AaBioWs.
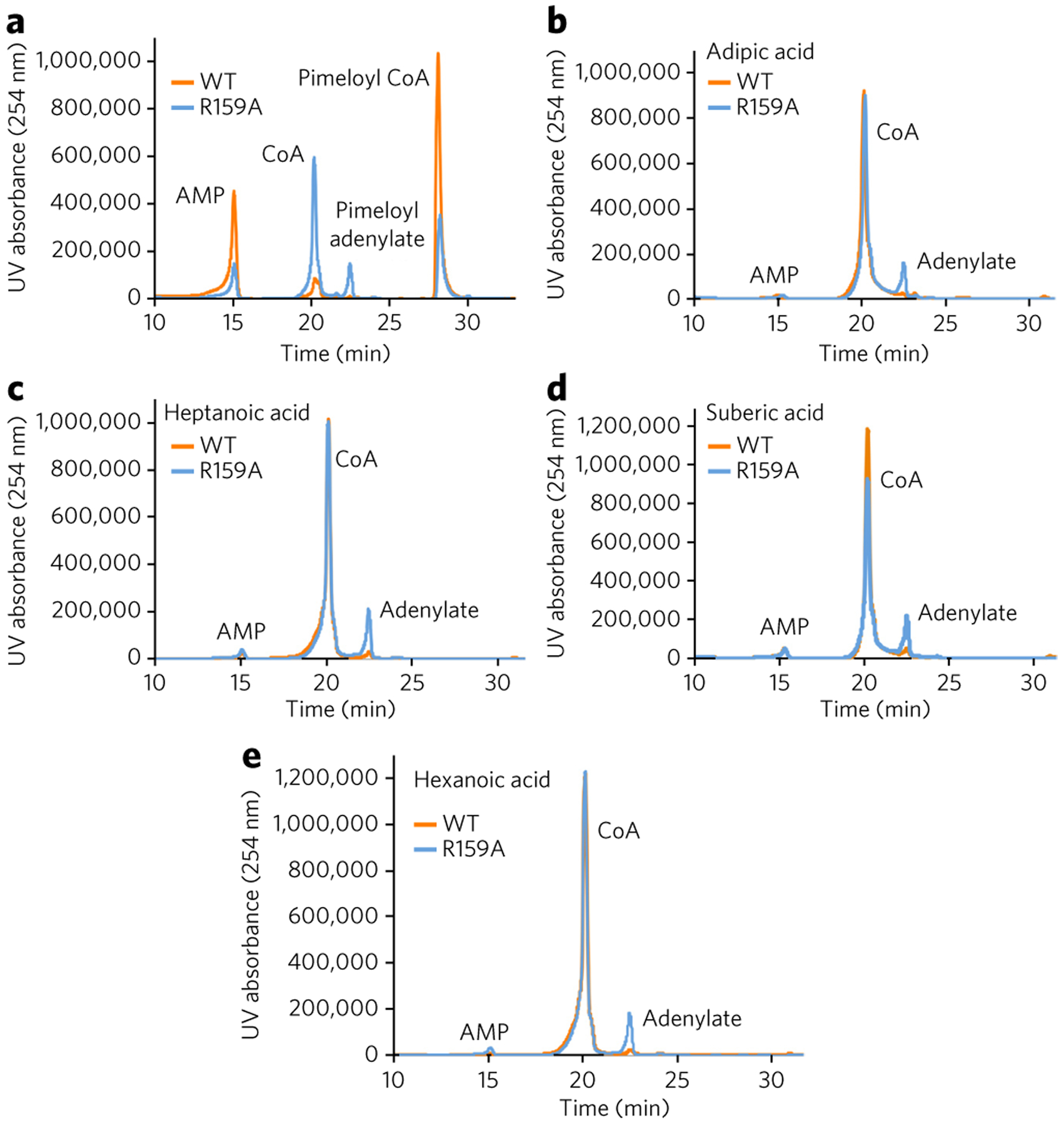
(a) Although the R159A variant can no longer proofread, the enzyme still retains ligase activity and can catalyze the formation of pimeloyl-CoA. (b–e) HPLC traces (absorbance at 254 nm for the AaBioW R159A mutant with different mono- and di-acid substrates: adipic acid (4; b), heptanoic acid (6; c), suberic acid (5; d), and hexanoic acid (7; e). Experiments were conducted in triplicate; data shown are representative of one to three measurements.
