Figure 3. Spectraplakins and Plakins.
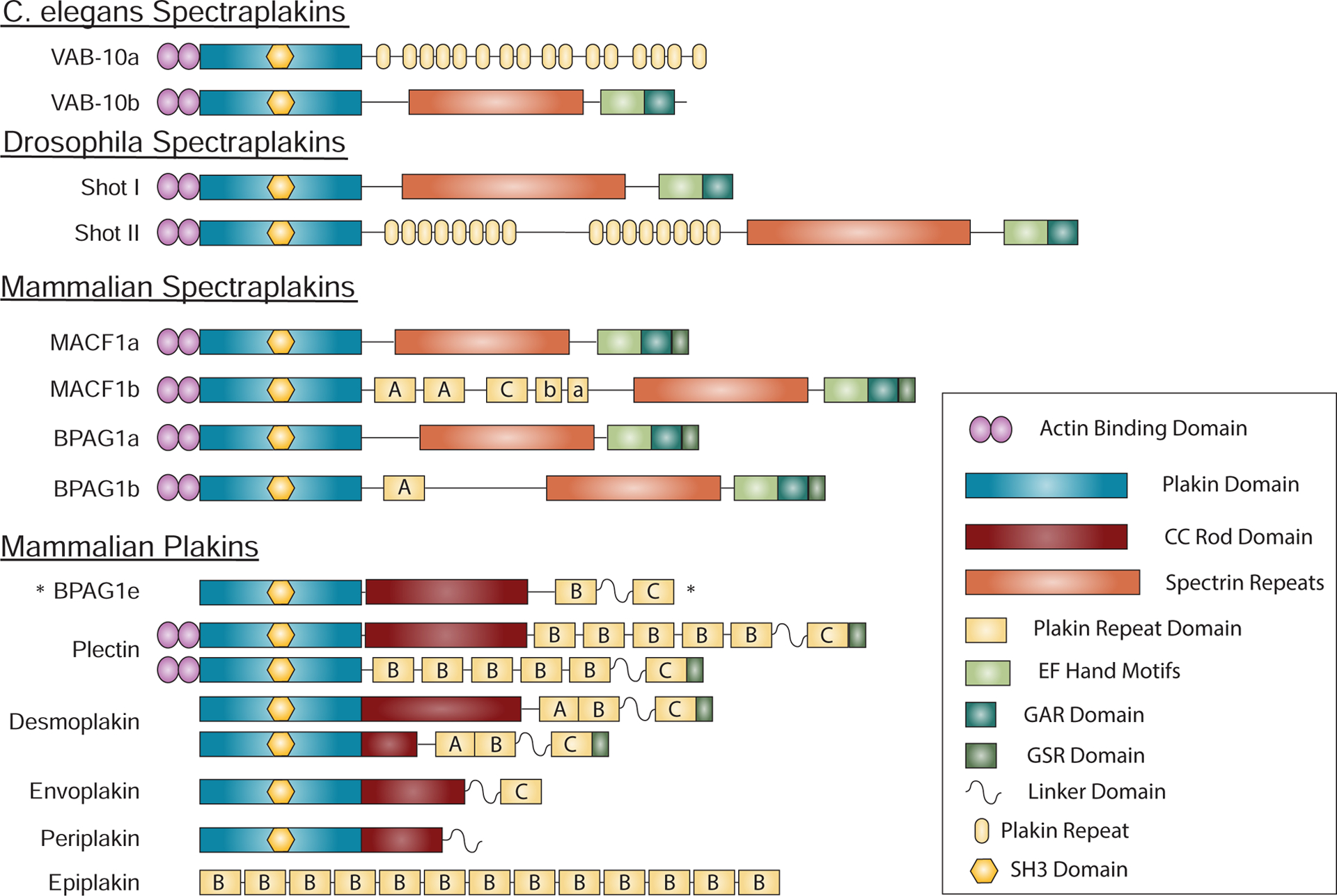
Spectraplakins are large proteins encompassing domains capable of interacting with all major cytoskeletal elements. These include actin binding domains (ABD), a plakin domain that targets proteins to junctions, plakin repeats that allow interactions with intermediate filaments, and EF hand, Gas2-related (GAR) and a regulatory glycine-serine-arginine rich (GSR) domains that harbor microtubule binding and regulatory functions. In mammalian spectraplakins and plakins, plakin repeats form plakin repeat domains (PRD) with three subtypes, A, B and C, and including a partial repeat (linker domain) between PRD B and C. The C. elegans and drosophila spectraplakins are encoded by the vab-10 and shot genes respectively, whereas mammalian spectraplakins are encoded by MACF1 and BPAG1/DST. The small number of spectraplakin genes give rise to a variety of spectraplakins by producing distinct splice variants. Plakins, present in vertebrates, likely arose from spectraplakins and allow linking of diverse junctions to intermediate filaments. Many plakins have lost the modules found in spectraplakins that allow interactions with actin and microtubules. BPAG1e, is unique in that it is classified as a plakin, while being a product of a spectraplakin gene. *This plakin is transcribed from from the bpag1 spectraplakin gene.
