Figure 7.
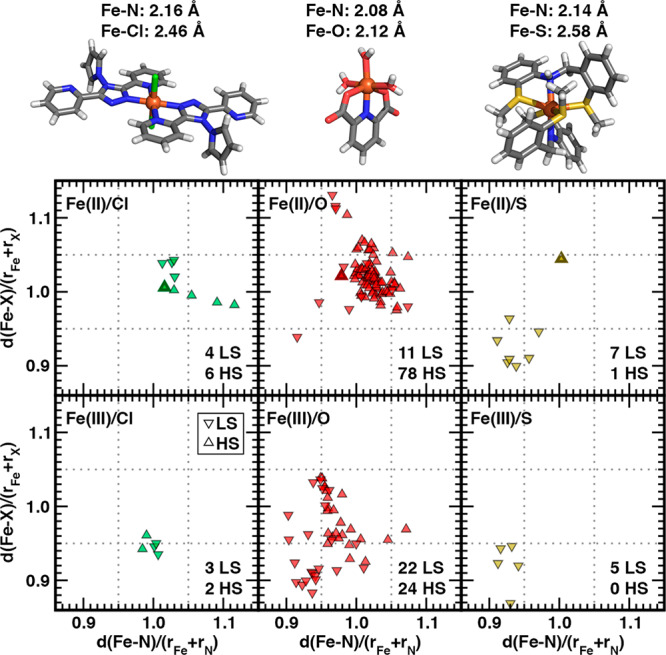
Fe–N vs Fe–X (X indicated according to inset in each pane) bond-length ratios computed relative to the sums of covalent radii for NX subset octahedral Fe(II) (top) and Fe(III) (bottom) complexes with N/Cl (left), N/O (middle), and N/S (right) coordinating atoms. Ratios of 0.95 and 1.05 are indicated by gray dotted lines. Only points for which spin-state assignment is confident are shown, and triangle down symbols indicate LS, whereas triangle up indicates HS. The total number with each spin assignment is shown in the bottom right corner of each pane. The Fe–N, Fe–X pair is computed from the average of all bonds of that type in the complex. Three representative HS Fe(II) complexes are shown at top and correspond to the only symbol that is solid filled with a dark colored border in each representative pane: N/Cl (left, CSD: POKNEJ), N/O (middle, CSD: DAQVEZ), N/S (right, CSD: ZERFEK). Structures are shown as sticks with carbon in gray, nitrogen in blue, hydrogen in white, chlorine in green, sulfur in yellow, oxygen in red, and iron in brown.
