Abstract
OBJECTIVE
To assess the prevalence of immunocompromised diagnoses among children with severe sepsis and septic shock, and to determine the association between immunocompromised diagnoses and clinical outcomes after adjustment for demographics and illness severity.
DESIGN
Retrospective multicenter cohort study.
SETTING
Eighty-three centers in the Virtual Pediatric Systems (VPS, LCC) database.
PATIENTS
Children with severe sepsis or septic shock admitted to a participating PICU between 1/1/2012 and 12/31/2016.
INTERVENTIONS
None.
MEASUREMENTS AND MAIN RESULTS
Across 83 centers, we identified 10,768 PICU admissions with an ICD-9-CM code for severe sepsis or septic shock; 3,021 of these patients (28%) had an immunocompromised diagnosis. To evaluate variation across centers and determine factors associated with PICU mortality, we used mixed-effect logistic regression models. Among patients without hematopoietic cell transplant, congenital immunodeficiency (aOR 1.90, 95% CI 1.24–2.92), multiple prior malignancies (aOR 1.86, 95% CI 1.15–2.99), and hemophagocytic lymphohistiocytosis (aOR 3.09, 95% CI 1.91–4.98) were associated with an increased odds of PICU mortality. Among patients with prior hematopoietic cell transplant, liquid malignancy (aOR 3.15, 95% CI 2.09–4.74), congenital immunodeficiency (aOR 6.94, 95% CI 3.84–12.53), multiple prior malignancies (aOR 3.54, 95% CI 1.80–6.95), and hemophagocytic lymphohistiocytosis (aOR 2.79, 95% CI 1.36–5.71) were associated with an increased odds of PICU mortality. PICU mortality varied significantly by center, and a higher mean number of sepsis patients per month in a center was associated with lower PICU mortality (aOR 0.94, 95% CI 0.90–0.98). PICU resource utilization varied by IC diagnosis and history of HCT, and among survivors IC patients have shorter median PICU LOS compared to patients without IC diagnoses (p<0.001).
CONCLUSIONS
Immunocompromised diagnoses are present in 28% of children with severe sepsis or septic shock. Multiple prior malignancies, hemophagocytic lymphohistiocytosis, congenital immunodeficiency, and hematopoietic cell transplant are independently associated with an increased odds of PICU mortality in children with severe sepsis or septic shock. Significant variation exists in PICU mortality among centers despite adjustment for immunocompromised diagnoses, known risk factors for sepsis-related mortality, and center-level sepsis volume.
Keywords: severe sepsis, septic shock, immunocompromised, pediatrics, epidemiology, critical care
INTRODUCTION
Sepsis is a leading cause of mortality in critically ill children and a common indication for admission to the pediatric intensive care unit (PICU) (1–5). The development of consensus guidelines and bundled sepsis care has led to improvements in sepsis mortality over the last decade, especially among previously healthy children (6–9).
Immunocompromised (IC) status is a biologically plausible and commonly acknowledged risk factor for sepsis, but the relationship between IC status and mortality in pediatric sepsis remains unclear. Previous studies have demonstrated increased sepsis-related mortality in subsets of patients with malignancy (10), congenital immunodeficiency (11), and hematopoietic cell transplant (HCT) (12–15), but prospective observational and interventional studies of pediatric sepsis have typically excluded IC patients (16, 17) or failed to capture sufficient data to assess the impact of IC status on sepsis outcomes (1–3, 18–20). Based on the available literature, it is difficult to draw generalizations about the epidemiology of severe sepsis in IC patients and therefore to assess clinically relevant risk factors and outcomes in this vulnerable population.
Pediatric patients with severe sepsis and septic shock who require critical care can be accurately identified in the Virtual Pediatric Systems (VPS, LLC) database using sepsis diagnosis codes for severe sepsis (995.92) and septic shock (785.52) (21). In the present study, we identify a large, multicenter cohort of patients with severe sepsis and septic shock using VPS, assess the prevalence of IC diagnoses among these patients, describe PICU resource utilization in subsets of IC patients, and determine the association between immunocompromised diagnoses and clinical outcomes after adjustment for demographics and illness severity. In our cohort, we hypothesized that the prevalence of IC diagnoses would be high, that IC status would be associated with increased PICU mortality, that clinical outcomes for patients with IC diagnoses would vary by center, and that patients with IC diagnoses would receive more intensive therapies during their PICU course.
MATERIALS AND METHODS
Study Design
After determination of exempt status by the IRB, we conducted an observational cohort study using data from the multicenter VPS database. All patient records in the VPS database during the study period (1/1/2012 to 12/31/2016) were queried for an ICD-9-CM code for severe sepsis (995.92) or septic shock (785.52) (3, 22), a method which we have previously shown to accurately identify patients with severe sepsis and septic shock in the VPS database (21). Patients aged <1 month or >18 years at PICU admission were excluded. Patients from low-volume centers which reported <6 cases of severe sepsis or septic shock per year were also excluded.
Data Collection
All available data were extracted from the VPS database, including demographic information, source of admission, coded diagnoses and procedures, severity of illness data [Paediatric Index of Mortality (PIM)-2 (23), Pediatric Risk of Mortality (PRISM)-III (24)], length of stay, and clinical outcome. The primary exposure was IC diagnosis defined by ICD-9-CM code (eTable 1); identified IC diagnoses included malignancy, solid organ transplant, congenital immunodeficiency, hemophagocytic lymphohistiocytosis, and aplastic anemia. History of hematopoietic cell transplant was also collected and analyzed as an effect modifier.
Hospital volume and experience with severe sepsis and septic shock were evaluated with a center-level variable defining the mean monthly volume of PICU patients with severe sepsis or septic shock, calculated for each site as the site total of severe sepsis/septic shock patients divided by the total number of months that site reported data to VPS.
Outcomes
The primary outcome was all-cause PICU mortality. Secondary outcomes included PICU length of stay (LOS) and rates of PICU resource utilization.
Statistical Analysis
PICU mortality was estimated with 95% confidence intervals and compared among cohorts using Fisher’s exact test. Differences in patient characteristics, PICU LOS, and PICU resource utilization were analyzed by Wilcoxon rank-sum test or Kruskal-Wallis test as appropriate for continuous variables and χ2 test for categorical variables. To evaluate variation in PICU mortality across centers and measure the association of patient characteristics with mortality, we used mixed-effect (ME) logistic regression models. Immunocompromised diagnoses were modeled as a single categorical variable; HCT was modeled as an effect modifier based on our prior study of sepsis-related mortality in HCT patients (15). The base ME model included no fixed effects and only a center-level random effect; the estimated variance of the random effect reflected the magnitude of the mortality variation across hospitals. In our ME model, a significant test of variance >0 suggests that the center-level variation is statistically significant. We subsequently added IC diagnoses and a priori patient factors previously associated with sepsis-related mortality – age, sex, source of admission, PRISM-III score (10, 14, 15) – to this model as fixed effects to assess if the variance of the center-level random effect remained significant. Finally, we added a center-level variable defining the mean monthly volume of PICU patients with severe sepsis or septic shock to the model as a fixed effect to assess the contribution of center volume to center-level variance in PICU mortality.
We also completed a sensitivity analysis to assess variation in PICU mortality among patients receiving intensive therapies. In this analysis, patients were stratified into three groups based on IC status – history of HCT, history of IC diagnosis without HCT, and no history of IC diagnosis. Rates and durations of intensive therapies, as well as associated mortality, are described and compared across groups. Analyses were performed using Stata/IC 15.1 (StataCorp, College Station, TX) with statistical significance defined as p<0.05.
RESULTS
Characteristics of Immunocompromised Patients with Severe Sepsis and Septic Shock
During the study period, we identified 10,768 PICU admissions with severe sepsis or septic shock from 83 PICUs. Immunocompromised diagnoses were identified in 28% of patients (3021/10,768) and 37% of nonsurvivors (502/1351); demographics, patient characteristics, and clinical outcomes are shown in Table 1, stratified by the presence of IC diagnosis. Compared to patients without IC diagnoses, IC patients had higher PICU mortality (17% vs 11%, p<0.001), higher PIM-2 and PRISM-III scores (p<0.001), and were more likely to be admitted from the inpatient floor (52% vs 22%, p<0.001). Conversely, IC patients have shorter median PICU LOS among survivors compared to patients without IC diagnoses (2.5 vs 5.0 days, p<0.001).
Table 1.
Characteristics of Patients with Severe Sepsis and Septic Shock
| Variable | Any IC Diagnosis (n=3,021) | No IC Diagnosis (n=7,747) | p a |
|---|---|---|---|
| Age Distribution, n (%) | |||
| 2 years to 5 years | 610 (20) | 1,385 (18) | <0.001 |
| 13 years to 18 years | 1,115 (37) | 2,279 (29) | |
| Male sex, n (%) | 1,642 (54) | 3,887 (50) | <0.001 |
| Non-Caucasian race, n (%) | 1,699 (53) | 4,122 (56) | <0.001 |
| PIM-2 probability of death, median [IQR] | 4.2 [1.2–6.1] | 1.4 [1.0–4.5] | <0.001 |
| PRISM-III score, median [IQR] | 11 [7–16] | 8 [3–14] | <0.001 |
| CPR prior to admission, n (%) | 43 (1.5) | 301 (4) | <0.001 |
| Fixed, dilated pupils on admission, n (%) | 20 (0.7) | 115 (1.5) | 0.003 |
| Admitted from floor, n (%) | 1,559 (52) | 1,667 (22) | <0.001 |
| Volume of Septic Patients per Month, median [IQR] | 4.8 [2.2–7.8] | 4.3 [1.7–7.6] | <0.001 |
| PICU LOS (d) among survivors, median [IQR] | 2.5 [1.3–6.8] | 5.0 [2.1–11.7] | <0.001 |
| PICU mortality, n (%) | 502 (17) | 849 (11) | <0.001 |
Wilcoxon rank-sum test for continuous variables; χ2 test for categorical variables.
IC: Immunocompromised; PIM-2: Paediatric Index of Mortality-2; PRISM-III: Pediatric Risk of Mortality-III; CPR: Cardiopulmonary Resuscitation; PICU: Pediatric Intensive Care Unit; LOS: Length of Stay; IQR: Interquartile Range.
Association of IC diagnoses with PICU Mortality
For our primary analysis, we measured the association of IC diagnosis with PICU mortality using a mixed effects model. We built the model starting with a center-level random effect and adding the following fixed effects: age, sex, source of admission, PRISM-III score, history of IC diagnoses, history of HCT, and center-level volume of septic patients. In our final ME model, several categories of IC diagnoses were associated with an increased odds of PICU mortality (Figure 1). Among patients without HCT, a diagnosis of congenital immunodeficiency (aOR 1.90, 95% CI 1.24–2.92), multiple prior malignancies (aOR 1.86, 95% CI 1.15–2.99), and hemophagocytic lymphohistiocytosis (aOR 3.09, 95% CI 1.91–4.98) were associated with an increased odds of all-cause PICU mortality. Among patients with prior HCT, a diagnosis of liquid malignancy (aOR 3.15, 95% CI 2.09–4.74), congenital immunodeficiency (aOR 6.94, 95% CI 3.84–12.53), multiple prior malignancies (aOR 3.54, 95% CI 1.80–6.95), and hemophagocytic lymphohistiocytosis (aOR 2.79, 95% CI 1.36–5.71) were associated with an increased odds of all-cause PICU mortality. Additionally, patients who received HCT without another underlying IC diagnosis had an increased odds of PICU mortality (aOR 4.74, 95% CI 2.56–8.77). Age at PICU admission, source of patient admission, PRISM-III score, and center-level volume of septic patients were all associated with PICU mortality in the final ME model; details of this model are shown in eTable 2.
Figure 1.
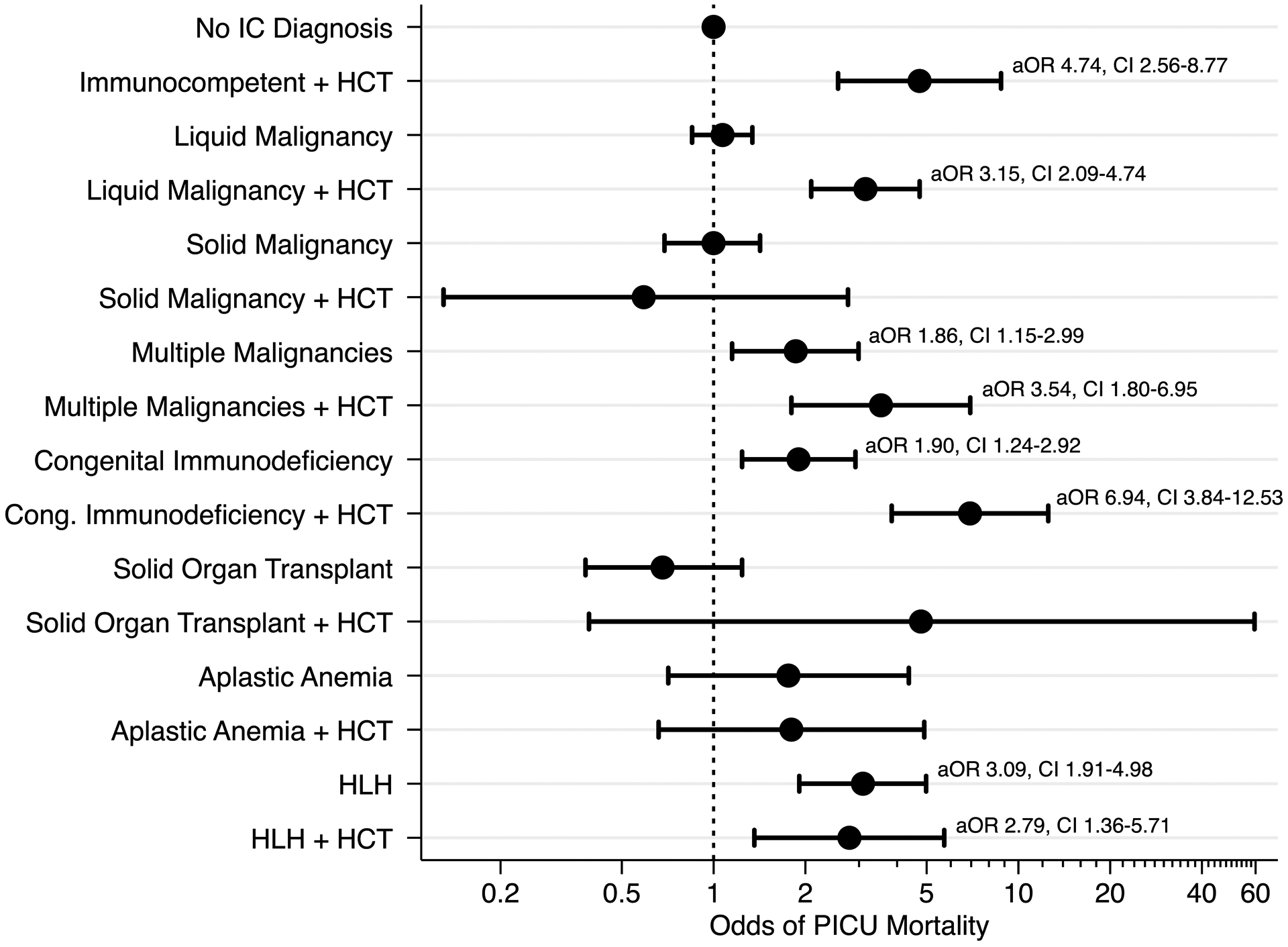
Association between IC diagnoses are PICU mortality among patients with severe sepsis or septic shock, after adjustment for a priori covariates in the final mixed effects model.
An exploratory analysis of the association between each specific IC diagnosis and PICU mortality using our final mixed-effects model is shown in eTable 3. In patients without prior HCT, Hodgkins lymphoma, myelodysplastic syndrome, multiple malignancies, severe combined immunodeficiency, and hemophagocytic lymphohistiocytosis were associated with an increased odds of PICU mortality. Among patients with prior HCT, acute lymphoid leukemia, acute myeloid leukemia, multiple malignancies, all subtypes of congenital immunodeficiency, and hemophagocytic lymphohistiocytosis were associated with an increased odds of PICU mortality.
Center-Level Variance in PICU Mortality
PICU mortality varied significantly by center from 0% to 50%, as shown in Figure 2a. A higher mean monthly volume of PICU patients with severe sepsis or septic shock was associated with lower PICU mortality in our final model (aOR 0.94, 95% CI 0.90–0.98), after adjustment for patient-level factors. The volume-outcome relationship between center-level volume of septic patients and PICU mortality is shown in Figure 2b. To further evaluate center-level variance, we utilized stepwise addition of patient- and center-level fixed effects in our ME model, as shown in Table 2. Center-level variance was significant in our base ME model. The addition of IC diagnoses and patient factors (age, gender, source of admission, PRISM-III score) to the model decreased center-level variance in PICU mortality, but this variance remained significant (p<0.001). The subsequent addition of mean center-level volume of septic patients further decreased this variance, but it remained statistically significant (p<0.001). Stratification of PICU mortality rate, PRISM-III score, and the discrimination of PRISM-III for mortality across deciles of center-level volume of septic patients did not demonstrate evidence of misclassification bias, as shown in eTable 4.
Figure 2.
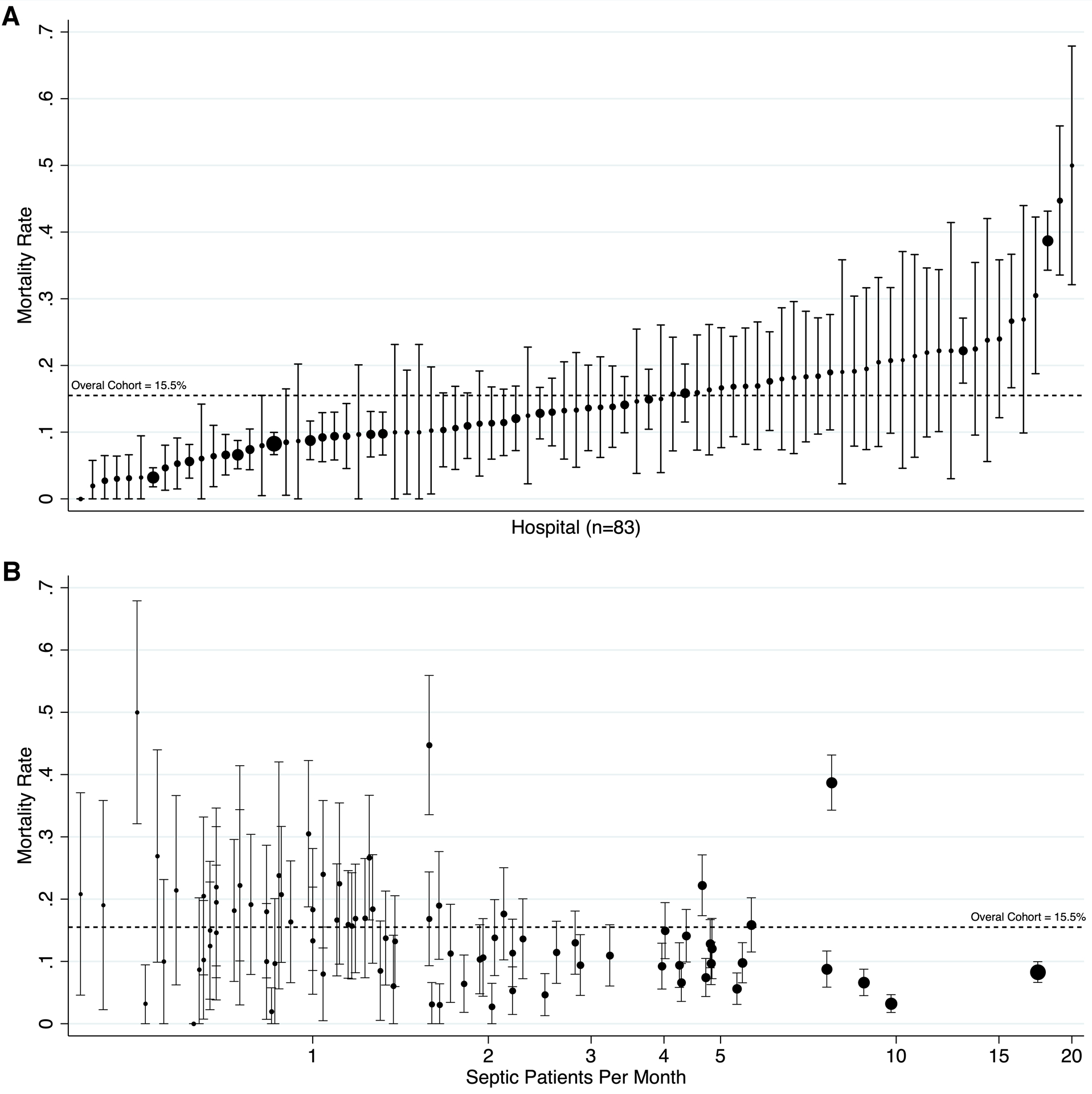
Center-level variation in PICU mortality for patients with severe sepsis or septic shock. Panel A depicts the mortality by hospital, ordered from lowest to highest. Panel B depicts the volume-outcome relationship between center-level volume of septic patients and PICU mortality. For both panels, the size of the point estimate corresponds to the volume of patients from each participating hospital. Error bars indicate the 95% confidence interval. The observed mortality rate for the entire cohort is indicated by a broken line at 15.5%.
Table 2.
Stepwise Assessment of Center-Level Variance in PICU Mortality
| Fixed Effects Included in Model | Variance of Hospital-Level Random Effect | 95% CI of the Variance | p |
|---|---|---|---|
| Hospital alone | 0.354 | 0.235 – 0.533 | <0.001 |
| Hospital + patient factors | 0.232 | 0.142 – 0.378 | <0.001 |
| Hospital + patient factors + center volume | 0.192 | 0.113 – 0.323 | <0.001 |
PICU Resource Utilization by Cohort of Immune Dysfunction
PICU resource utilization varied by IC diagnosis and history of HCT. Rates of arterial line placement, invasive mechanical ventilation, hemodialysis, and extracorporeal support are shown in Table 3, stratified by cohort of IC patient. A significant proportion of patients with HCT (42%) and IC patients without HCT (55%) received no invasive therapies during their PICU admission; their mortality rates were low. Rates of utilization of all PICU therapies varied significantly among groups (p<0.001). Among patients receiving arterial line placement, mechanical ventilation, or hemodialysis, mortality rates varied significantly across cohorts (all p<0.001) and were highest among HCT patients. ECMO use was uncommon in all three cohorts. Among survivors, duration of arterial line placement, invasive mechanical ventilation, and hemodialysis did not vary among groups.
Table 3.
PICU Resource Utilization by Group
| Variable | HCT (n=503) | IC, no HCT (n=2,518) | Not IC (n=7,747) | p a |
|---|---|---|---|---|
| Patients who received no invasive therapies | ||||
| No arterial line, invasive mechanical ventilation, dialysis, or ECMO, n (%) | 211 (42) | 1,397 (55) | 2,615 (34) | <0.001 |
| PICU mortality, n (%) | 13 (6) | 19 (1.4) | 32 (1.2) | <0.001 |
| Patients who received any invasive therapies | ||||
| Arterial line, invasive mechanical ventilation, dialysis, or ECMO, n (%) | 292 (58) | 1,121 (45) | 5,132 (66) | <0.001 |
| PICU mortality, n (%) | 145 (50) | 325 (29) | 817 (16) | <0.001 |
| Patients who received arterial line placement | ||||
| Arterial line placement, n (%) | 224 (44) | 936 (37) | 3,751 (48) | <0.001 |
| PICU mortality, n (%) | 108 (48) | 263 (28) | 614 (16) | <0.001 |
| Arterial line duration among survivors (d), median [IQR] | 4.6 [1.7–9.0] | 3.9 [1.9–8.0] | 4.0 [2.0–7.4] | 0.65 |
| Patients who received invasive mechanical ventilation | ||||
| Invasive mechanical ventilation initiation, n (%) | 237 (47) | 880 (35) | 4,566 (59) | <0.001 |
| PICU mortality, n (%) | 137 (58) | 310 (35) | 802 (18) | <0.001 |
| Invasive mechanical ventilation duration among survivors (d), median [IQR] | 3.7 [1.6–7.9] | 4.6 [1.9–8.8] | 4.9 [2.5–8.8] | 0.07 |
| Patients who received hemodialysis | ||||
| Hemodialysis initiation, n (%) | 78 (16) | 240 (10) | 455 (6) | <0.001 |
| PICU mortality, n (%) | 49 (63) | 125 (52) | 120 (26) | <0.001 |
| Hemodialysis duration among survivors (d), median [IQR] | 3.7 [1.7–8.6] | 5.0 [2.3–10.0] | 4.3 [1.8–9.2] | 0.71 |
| Patients who received extracorporeal membrane oxygenation (ECMO) | ||||
| ECMO initiation, n (%) | 5 (1) | 38 (2) | 215 (3) | <0.001 |
| PICU mortality, n (%) | 3 (60) | 24 (63) | 108 (50) | 0.32 |
| ECMO duration among survivors (d), median [IQR] | 8.0 [5.6–10.3] | 5.9 [2.8–8.9] | 6.1 [3.2–10.4] | 0.78 |
χ2 and Fisher’s exact test for categorical variables as appropriate.
PICU: Pediatric Intensive Care Unit; HCT: Hematopoietic Cell Transplant; IC: Immunocompromised; ECMO: Extracorporeal Membrane Oxygenation; IQR: Interquartile Range.
DISCUSSION
This is the first large, multicenter cohort study designed to evaluate the association between immunocompromised status and mortality in pediatric severe sepsis and septic shock. Using a well-defined cohort of patients from the VPS database, we found that 28% of children with severe sepsis or septic shock have an underlying immunocompromising diagnosis, and that a history of multiple prior malignancies, hemophagocytic lymphohistiocytosis, congenital immunodeficiency, and hematopoietic cell transplant are associated with an increased risk of sepsis-related mortality. Through our adjusted analysis in which we accounted for patient age, sex, source of admission, PRISM-III score, and center-level volume of septic patients, we identified that several IC diagnoses are not associated with increased sepsis-related mortality despite being traditionally thought of as high-risk diagnoses, specifically hematologic malignancy without prior HCT as well as history of solid organ transplant. We also demonstrated significant variation in PICU resource utilization by diagnosis and substantial variation in sepsis-related mortality across centers, in which higher center-level volume of septic patients is associated with significantly lower PICU mortality, even after adjustment for severity of illness and IC status.
In our primary analysis, we identified several IC phenotypes associated with an increased odds of PICU mortality. Among patients without HCT, a history of multiple malignancies, congenital immunodeficiency, and hemophagocytic lymphohistiocytosis were associated with increased sepsis-related mortality. While HCT is a known, significant risk factor for sepsis-related mortality, these specific IC diagnoses also confer an increased risk in the absence of HCT and may warrant special consideration due to their heightened vulnerability to sepsis-related PICU mortality. For patients with previous HCT, the association between underlying IC diagnosis and PICU mortality was variable. Underlying conditions typically treated with allogeneic HCT (e.g. liquid malignancy, congenital immunodeficiency) demonstrated a robust association between HCT and sepsis-related mortality, while conditions typically treated with autologous HCT (e.g. solid malignancy) did not demonstrate an association between HCT and sepsis-related mortality.
Our primary analysis yields several novel insights into specific IC phenotypes which are relevant to both clinicians and sepsis researchers. First, IC diagnoses are very common among children with severe sepsis and septic shock, present in 28% of PICU patients with severe sepsis or septic shock and 37% of sepsis-related PICU mortalities. Second, our results confirm that a history of HCT is a major risk factor for PICU mortality among patients with sepsis, and the pattern of this association is strongest for diagnoses typically treated with allogeneic HCT. These findings are congruent with prior reports which have demonstrated high levels of morbidity and mortality associated with sepsis in HCT patients (14, 15). Third, we found that in the absence of HCT, septic patients with liquid and solid malignancy do not have an increased odds of PICU mortality. Many prior studies have demonstrated increased sepsis-related mortality in patients with liquid malignancies; in light of our present results, the mortality risk of this population is likely attributable to the prevalence of HCT among patients with liquid malignancy. This finding is consistent with two previous studies of sepsis which have included oncology patients but excluded HCT patients from analysis (12, 25). Fourth, IC patients were more likely to be admitted from the general inpatient ward than patients without IC diagnoses. Our group has previously demonstrated this association among HCT patients with sepsis (15), and these results confirm that the majority of episodes of sepsis among IC patients occur during acute inpatient hospitalization. Proactive surveillance and early intervention in hospitalized patients with sepsis remain an opportunity for improvement given their risk of sepsis-related morbidity and mortality, and specialized care teams with expertise in pediatric onco-critical care may have an important role in the clinical management of these patients, especially in high-volume centers. Finally, our group has previously shown that septic IC patients without HCT represent an intermediate risk phenotype (15); the present study demonstrates that clinical outcomes among IC patients are heterogeneous and suggests that only select diagnoses actually confer an increased risk of sepsis-related mortality.
We found that PICU mortality varied significantly across the 83 centers in our cohort. While some variance was explained by patient-level factors, including IC diagnoses and illness severity scores, as well as the center-level volume of septic patients, significant variance among centers remained after both adjustments. Higher center-level volume of septic patients was independently associated with a decreased odds of PICU mortality. This association could reflect higher-quality sepsis care in high-volume centers, or more liberal coding behavior for severe sepsis and septic shock among those centers, leading to a lower mortality rate by including patients with less organ dysfunction in the identified cohort of septic patients. To address this concern, we included admission illness severity as a covariate in our ME model using PRISM-III, and while PRISM-III showed good discrimination for mortality across deciles of center-level volume of septic patients, it is possible that this adjustment fails to completely account for illness severity.
In analysis of our secondary outcomes, we identified several associations between PICU resource utilization and IC phenotypes. Many IC patients with severe sepsis and septic shock did not receive invasive therapies during their PICU admission; this finding may reflect a lower threshold to seek critical care for these patients due to a perceived vulnerable status. Among patients who receive invasive therapies, mortality rates are significantly higher among IC patients than patients without IC diagnoses. HCT patients requiring arterial line placement, invasive mechanical ventilation, or hemodialysis are very high-risk patients, with a combined PICU mortality rate of 50% in this cohort. Finally, the use of extracorporeal support for severe sepsis and septic shock remains uncommon, a finding consistent with a previous study in which only 2% of septic patients received extracorporeal membrane oxygenation (26).
While retrospective studies of sepsis epidemiology have inherent limitations, our study has several notable strengths. We have previously demonstrated that the multicenter VPS dataset can be used to identify an accurate cohort of patients with sepsis and high disease severity (21), and in the present analysis we have leveraged that cohort to yield important new insights into sepsis-related mortality among IC patients. Unlike administrative datasets, data in VPS is extracted by expert, trained coders according to standard data definitions subject to quarterly inter-rater reliability testing. This dataset also includes required reporting of PICU procedures and robust severity of illness data, which allows for careful selection and adjustment for covariates. Despite these strengths, there are important limitations which must be considered when interpreting these results. First, IC phenotypes were identified by diagnosis code, and thus no information regarding current disease status, severity of clinical phenotype, stage of malignancy, and concurrent disease-modifying therapies were available for analysis. Due to this data limitation, we were also unable to identify and assess patients who are immunocompromised due to chronic immunosuppressive therapies, and IC phenotypes could not be confirmed with clinical criteria. Second, information regarding indication for HCT, conditioning regimen, transplant type, source of cells, antibiotic exposures, and transplant-related complications is unavailable in VPS. Inclusion of these variables would allow for further in-depth risk stratification in this important group of patients known to be at high risk of sepsis-related mortality. Finally, we are unable to definitively assess the impact of variance in sepsis coding behavior across VPS sites, which may influence our center-level analysis of PICU mortality.
As the science of pediatric sepsis continues to develop, we must pay careful attention to the high-risk cohort of children with IC conditions. In our present analysis, 37% of sepsis deaths occurred in children with an IC diagnosis. These children are typically excluded from observational and interventional sepsis studies due to concerns of confounding and heterogeneity. However, their altered immunobiology and center-level variance in care practices provide opportunity for important, novel insights into the fundamental biology of this challenging clinical syndrome. Additional insights into IC sepsis epidemiology could be accomplished through the merger of VPS with existing large pediatric registries, including the Pediatric Health Information System (PHIS) database (26) and the Center for International Blood and Marrow Transplant Research (CIBMTR) registry (27). Further, once these high-risk sepsis phenotypes are clearly established, targeted prospective studies of innate and adaptive immune function are imperative to improve our understanding of sepsis pathobiology and impact the care provided to these highest-risk critically ill children.
CONCLUSIONS
In this large, multicenter study of pediatric severe sepsis, IC diagnoses were present in 28% of patients with sepsis and 39% of patients dying with sepsis. After adjustment for measured confounders, multiple prior malignancies, hemophagocytic lymphohistiocytosis, congenital immunodeficiency, and hematopoietic cell transplant were associated with an increased odds of PICU mortality. There was significant variation in PICU mortality among centers despite adjustment for IC diagnoses, center-level volume of septic patients, and known risk factors for sepsis-related mortality. Higher center-level volume of septic patients was associated with decreased odds of PICU mortality. Further research into IC sepsis epidemiology and immunobiology is critical to improving survival in this heterogeneous, high-risk cohort of patients.
Supplementary Material
eTable 1. Classification of Immunocompromised Diagnoses and Associated ICD-9-CM Codes
eTable 2. Details of Covariate Association with Mortality in Final Mixed-Effect Model
eTable 3. Detailed Analysis of Specific IC Diagnoses in Final Mixed-Effect Model
eTable 4. Stratification of PICU mortality rate, PRISM-III score, and discrimination of PRISM-III for PICU mortality across deciles of center-level volume of septic patients
ACKNOWLEDGEMENTS
VPS data was provided by Virtual Pediatric Systems, LLC. No endorsement or editorial restriction of the interpretation of these data or opinions of the authors has been implied or stated. This manuscript has been reviewed by the VPS Research Committee.
Conflicts of Interest and Source of Funding:
Financial support was provided by the Endowed Chair, Department of Anesthesiology and Critical Care, Children’s Hospital of Philadelphia and The University of Pennsylvania Perelman School of Medicine. Dr. Lindell is also supported by the Thrasher Research Fund. Dr. Weiss is also supported by NIGMS K23-GM110496. Dr. Nishisaki is also supported by NICHD R21-HD089151 and AHRQ R18 HS024511. For the remaining authors, no conflicts were declared.
Footnotes
Copyright form disclosure:
Dr. Lindell is supported by the Thrasher Research Fund. Dr. Weiss is supported by NIGMS K23-GM110496. Dr. Nishisaki is supported by NICHD R21-HD089151 and AHRQ R18 HS024511. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Watson RS, Carcillo JA, Linde-Zwirble WT, et al. : The Epidemiology of Severe Sepsis in Children in the United States. Am J Respir Crit Care Med 2003; 167:695–701 [DOI] [PubMed] [Google Scholar]
- 2.Ruth A, McCracken CE, Fortenberry JD, et al. : Pediatric severe sepsis: current trends and outcomes from the Pediatric Health Information Systems database. Pediatric Critical Care Medicine 2014; 15:828–838 [DOI] [PubMed] [Google Scholar]
- 3.Balamuth F, Weiss SL, Neuman MI, et al. : Pediatric severe sepsis in U.S. children’s hospitals. Pediatric Critical Care Medicine 2014; 15:798–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss SL, Fitzgerald JC, Pappachan J, et al. : Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015; 191:1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlapbach LJ, Straney L, Alexander J, et al. : Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002–13: a multicentre retrospective cohort study. Lancet Infect Dis 2015; 15:46–54 [DOI] [PubMed] [Google Scholar]
- 6.Brierley J, Carcillo JA, Choong K, et al. : Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine*. Critical Care Medicine 2009; 37:666–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans IVR, Phillips GS, Alpern ER, et al. : Association Between the New York Sepsis Care Mandate and In-Hospital Mortality for Pediatric Sepsis. JAMA 2018; 320:358–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Workman JK, Ames SG, Reeder RW, et al. : Treatment of Pediatric Septic Shock With the Surviving Sepsis Campaign Guidelines and PICU Patient Outcomes. Pediatric Critical Care Medicine 2016; 17:e451–e458 [DOI] [PubMed] [Google Scholar]
- 9.Han YY, Carcillo JA, Dragotta MA, et al. : Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics 2003; 112:793–799 [DOI] [PubMed] [Google Scholar]
- 10.Zinter MS, DuBois SG, Spicer A, et al. : Pediatric cancer type predicts infection rate, need for critical care intervention, and mortality in the pediatric intensive care unit. Intensive Care Med 2014; 40:1536–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faraci M, Bagnasco F, Giardino S, et al. : Intensive care unit admission in children with malignant or nonmalignant disease: incidence, outcome, and prognostic factors: a single-center experience. J Pediatr Hematol Oncol 2014; 36:e403–9 [DOI] [PubMed] [Google Scholar]
- 12.Kutko MC, Calarco MP, Flaherty MB, et al. : Mortality rates in pediatric septic shock with and without multiple organ system failure. Pediatric Critical Care Medicine 2003; 4:333–337 [DOI] [PubMed] [Google Scholar]
- 13.Fiser RT, West NK, Bush AJ, et al. : Outcome of severe sepsis in pediatric oncology patients. Pediatric Critical Care Medicine 2005; 6:531–536 [DOI] [PubMed] [Google Scholar]
- 14.Zinter MS, Dvorak CC, Spicer A, et al. : New Insights Into Multicenter PICU Mortality Among Pediatric Hematopoietic Stem Cell Transplant Patients. Critical Care Medicine 2015; 43:1986–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindell RB, Gertz SJ, Rowan CM, et al. : High Levels of Morbidity and Mortality Among Pediatric Hematopoietic Cell Transplant Recipients With Severe Sepsis: Insights From the Sepsis PRevalence, OUtcomes, and Therapies International Point Prevalence Study. Pediatric Critical Care Medicine 2017; 18:1114–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadel S, Goldstein B, Williams MD, et al. : Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet 2007; 369:836–843 [DOI] [PubMed] [Google Scholar]
- 17.Downes KJ, Weiss SL, Gerber JS, et al. : A Pragmatic Biomarker-Driven Algorithm to Guide Antibiotic Use in the Pediatric Intensive Care Unit: The Optimizing Antibiotic Strategies in Sepsis (OASIS) Study. J Pediatric Infect Dis Soc 2016; 61:piw023–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Oliveira CF, de Oliveira DSF, Gottschald AFC, et al. : ACCM/PALS haemodynamic support guidelines for paediatric septic shock: an outcomes comparison with and without monitoring central venous oxygen saturation. Intensive Care Med 2008; 34:1065–1075 [DOI] [PubMed] [Google Scholar]
- 19.Choong K, Bohn D, Fraser DD, et al. : Vasopressin in pediatric vasodilatory shock: a multicenter randomized controlled trial. Am J Respir Crit Care Med 2009; 180:632–639 [DOI] [PubMed] [Google Scholar]
- 20.Ventura AMC, Shieh HH, Bousso A, et al. : Double-Blind Prospective Randomized Controlled Trial of Dopamine Versus Epinephrine as First-Line Vasoactive Drugs in Pediatric Septic Shock. Critical Care Medicine 2015; 43:2292–2302 [DOI] [PubMed] [Google Scholar]
- 21.Lindell RB, Nishisaki A, Weiss SL, et al. : Comparison of Methods for Identification of Pediatric Severe Sepsis and Septic Shock in the Virtual Pediatric Systems Database. Critical Care Medicine 2019; 47:e129–e135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balamuth F, Weiss SL, Hall M, et al. : Identifying Pediatric Severe Sepsis and Septic Shock: Accuracy of Diagnosis Codes. J Pediatr 2015; 167:1295–300.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slater A, Shann F, Pearson G, et al. : PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med 2003; 29:278–285 [DOI] [PubMed] [Google Scholar]
- 24.Pollack MM, Patel KM, Ruttimann UE: PRISM III: an updated Pediatric Risk of Mortality score. Critical Care Medicine 1996; 24:743–752 [DOI] [PubMed] [Google Scholar]
- 25.Maude SL, Fitzgerald JC, Fisher BT, et al. : Outcome of pediatric acute myeloid leukemia patients receiving intensive care in the United States. Pediatric Critical Care Medicine 2014; 15:112–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruth A, McCracken CE, Fortenberry JD, et al. : Extracorporeal therapies in pediatric severe sepsis: findings from the pediatric health-care information system. Crit Care 2015; 19:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horowitz M: The role of registries in facilitating clinical research in BMT: examples from the Center for International Blood and Marrow Transplant Research. Bone Marrow Transplant 2008; 42 Suppl 1:S1–S2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Classification of Immunocompromised Diagnoses and Associated ICD-9-CM Codes
eTable 2. Details of Covariate Association with Mortality in Final Mixed-Effect Model
eTable 3. Detailed Analysis of Specific IC Diagnoses in Final Mixed-Effect Model
eTable 4. Stratification of PICU mortality rate, PRISM-III score, and discrimination of PRISM-III for PICU mortality across deciles of center-level volume of septic patients


