Abstract
Background:
Cardiopulmonary bypass may be associated with post-operative neurocognitive dysfunction (NCD); however, risk factors have not been clearly identified. We hypothesize that lower hematocrit levels are correlated with post-operative neurocognitive dysfunction.
Methods:
Thirty patients underwent cardiac operations utilizing cardiopulmonary bypass and screening for neurocognitive dysfunction pre-operatively and on post-operative day four (POD4). Patients were analyzed according to hematocrit pre-operatively, 6 hours post-operatively and on POD4, and whether they received intra or post-operative transfusions of packed red blood cells. Neurocognitive data is presented as a difference in Repeatable Battery for the Assessment of Neuropsychological Status standardized score from baseline to POD4 and analyzed by unpaired two tailed Spearman test.
Results:
There was a significant correlation between patients with lower hematocrit prior to surgery and a decline in neurocognitive function at POD4 (p<0.05). All patients experienced a decrease in hematocrit during their hospital stay; but the hematocrit 6 hours post-operatively and POD4 did not impact cognition. Receiving a transfusion was also not associated with NCD. Patients with low hematocrit pre-operatively had a consistently lower hematocrit throughout their stay. Prolonged total length of stay was also significantly associated with neurocognitive decline.
Conclusions:
A lower preoperative hematocrit and prolonged length of hospital stay are correlated with neurocognitive decline following cardiac surgery utilizing cardiopulmonary bypass.
Graphical Abstract
We have found that a lower hematocrit prior to cardiopulmonary bypass and longer hospital stay are associated with neurocognitive decline. The importance of this is finding is that neurocognitive decline has not been a considered morbidity in transfusion studies that have changed the practice in surgery.
Introduction
Although advances in cardiac surgery and anesthesia have decreased the morbidity and mortality associated with cardiopulmonary bypass (CPB), neurocognitive decline (NCD) remains a significant consequence of these operations.1 This decline can be both acute and chronic, with 24–53% of patients affected at discharge and up to 42% at five years.23 The relative roles of CPB, trauma of surgery, aging, diabetes, hypertension and cardiovascular disease on long term NCD after cardiac surgery remain debatable. Despite initial reports suggesting that patients were unaware of their own decline in cognition, a new study reports patients are in fact aware of their decline in cognitive function, primarily in memory, both at three months and a year after surgery.4 This becomes a significant contributor to patients’ decreased quality of life after their operation, with cognitive function at discharge being a significant predictor of long-term function.2
In our study, we hypothesized that there is an association between anemia, transfusion and early (post-operative day four [POD4]) NCD and sought to better characterize patient features associated with increased risk of NCD that in turn may be targetable for perioperative treatment and management.
Patients and Methods
Patient Enrollment
Patients were enrolled in our single-institution, prospective cohort study at Rhode Island Hospital. All patients were scheduled for primary CABG, valve replacement (aortic or mitral), or a combination of these procedures. Patients underwent a sternotomy except for one patient who underwent a minimally invasive aortic valve replacement. All the operations utilized CPB. Only patients who were native English speakers were included in the study. Thirty patients were included in the analysis based on their completion of the neurocognitive assessment on POD4.
Patients were excluded if they had suffered from a stroke within the past year, chronic renal failure (defined as creatinine greater than 2.0mg/dL), hepatic disease (defined as abnormal liver function tests or cirrhosis), severe neurological deficits, a heavily calcified aorta, high grade carotid artery stenosis (>70%), severely impaired vision and those who underwent an aortic arch or root procedure. All patients provided informed consent, and all procedures were approved by the Institutional Review Board of Rhode Island Hospital in Providence, RI. IRB Registration #s: RIH IRB 1 – 00000396, RIH IRB 2 – 00004624, TMH IRB – 00000482
Patients underwent induction of general anesthesia and placement of invasive monitoring lines followed by either a midline sternotomy or right anterior thoracotomy for the minimally invasive case per standard cardiac surgical technique. Operations were performed after heparinization and under conditions of CPB. A hypothermic blood-based cardioplegia solution (8°C, 4:1 mixture of oxygenated blood and hyperkalemic crystalloid solution; delivered antegrade into the aortic root or retrograde into the coronary sinus.
Transfusion Technique
Intraoperative transfusion was at the discretion of the treating team. The postoperative transfusion protocol at Rhode Island Hospital is transfusion with one unit of packed red blood cells if hematocrit is ≤ 21%. Patients are also transfused if their hematocrit is between 22 and 27% and the patient is either on norepinephrine > 10mcg/kg/min or vasopressin 0.04 U/min, mean arterial pressure < 65mmHg, has a cardiac index < 2, or mixed venous O2 saturation less then 60% despite CVP or PA diastolic pressure being above 12 mm Hg.
Neurocognitive Assessment
All patients were administered a Repeatable Battery of Assessment of Neuropsychological Status (RBANS) within a week prior to and four days after their operation. The test assesses global cognition: immediate and delayed memory abilities, attention, language, visuospatial function and has been proven to detect even mild impairment.5
The RBANS has two forms that consist of the same tests, but with variation in the specific words, stories, pictures, et cetera used. One form was administered prior to the operation and one after. The results were scored according to the corresponding scoring guide to calculate both a raw and scaled score for each cognitive domain and total score. The raw cognitive domain score assigns a value to the participant’s performance based on a normative sample of healthy individuals on which RBANS is based. The individual cognitive domain scores are based on a gaussian distribution of the original population with mean of 100 and standard deviation of 15. The raw total score is the sum of individual cognitive domain scores, mean is 500. The scaled score considers the form, age and gender to assign a score based on the normal distribution of the healthy population of the same age and gender, where the mean is 10 and 3 is the standard deviation. The total scaled score is based on the normalized distribution of the original population studied of the same age and gender as the person being evaluated, where 100 is the mean and the standard deviation is 15.6 Examiners were native English speakers and trained by neuropsychologists to administer the RBANS. The same individual administered both batteries.
Statistical Analysis
The study examined participants’ difference in standardized scores on the RBANS from baseline (before surgery) to POD4. This score was analyzed in a univariate analysis against varying characteristics of the surgery, labs prior to the operation, six hours and four days after the operation, and interventions during their hospital stay, collected from patients’ medical records. The two-tailed Spearman Test was used to analyze continuous variables. The unpaired Mann-Whitney U Test was used for Boolean variables. For comparison of function of individual cognitive domains between baseline and POD4, the paired Wilcoxon sign-rank test was used.
Results
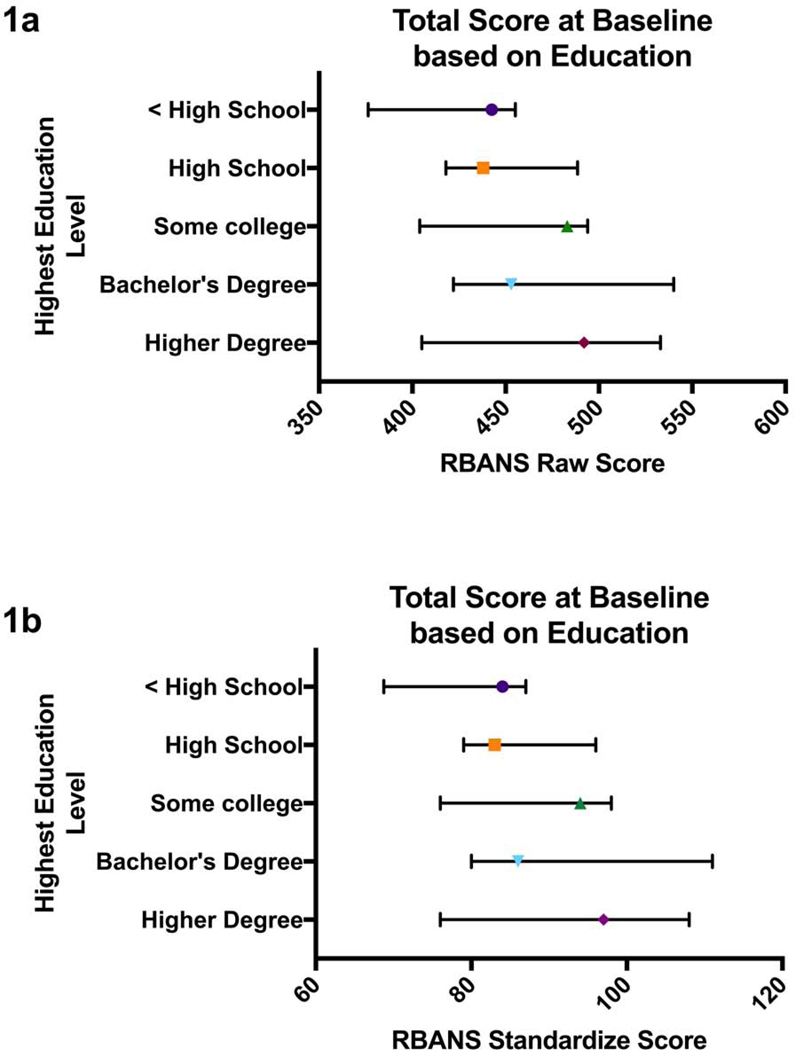
Table 1 contains the characteristics of the patient population in the study. Notably, the majority of patients in our study were white, male and had at least a high school diploma. Most of the patients had a diagnosis of hypertension, hyperlipidemia, and diabetes. Over half of the population had a history smoking and did not report drinking alcohol to excess. Educational level did not correlate with success on any one cognitive domain. Both total and total scaled score were greater with more education (Figure 1).
Table 1:
Patient Demographics
| Category | # of patients (percentage) | |
|---|---|---|
| Mean age ± SD | 67.3 years ± 9.7 | − |
| Gender | Male | 22 (73%) |
| Female | 8 (27%) | |
| Race | White | 28 (93%) |
| Black or African American | 1 (3%) | |
| Native American | 1 (3%) | |
| Education Completed | High School Diploma | 24 (80%) |
| Some College | 15 (50%) | |
| Bachelor Degree | 8 (27%) | |
| Master/Doctoral Degree | 5 (17%) | |
| Operation | CABG only | 22 (73.3%) |
| AVR only | 3 (10.0%) | |
| CABG + MVR | 3 (10.0%) | |
| CABG + AVR | 1 (3.3%) | |
| Minimally invasive MVR | 1 (3.3%) | |
| Comorbidities | Diabetes | 15 (50%) |
| Hypertension | 27 (90%) | |
| Hyperlipidemia | 25 (83%) | |
| Heart Failure | 8 (27%) | |
| Lung Disease | 1 (3%) | |
| Tobacco use | Current smoker | 5 (17%) |
| Former smoker | 15 (50%) | |
| Never smoked | 10 (33%) | |
These are patient demographics, educational level, prevalence of comorbidities, incidence of medically treated comorbidities, smoking history and alcohol use information about the patients in the study.
Figure 1.
Figure 1a: Patients’ total RBANS raw score at baseline stratified by education level. 1b: Patients’ total RBANS standardized score at baseline stratified by education level. Median and interquartile ranges are marked on the graph.
There was no association between preoperative demographic data or surgical characteristics and NCD. (Table 2). There was a 53% (16/30) incidence to atrial fibrillation after surgery. There was no relationship between the development of atrial fibrillation and decrease in cognitive function on POD4 (p=0.76, Table 2). Two patients were taken back to the OR several hours after surgery to control bleeding. Other continuous variables associated with the surgery were not associated with any change in cognitive function including patient age, total operative time, time on CPB, aortic cross-clamp time, preoperative blood pressure, intra-operative glucose control, hematocrit nadir during bypass, blood pressures during bypass (Table 3). Patients with a longer hospital stay (r= −0.45, p=0.01) were more likely to have a decrease in cognitive function on POD4. We found that classifying patient’s six-hour and POD4 laboratory characteristics were not associated with any significant difference in their change in neurocognitive function.
Table 2:
Correlations of Demographic and Surgical Characteristics with Neurocognitive Decline Dichotomous Variables
| Demographics and Surgery Characteristics | Groups | Median Change in RBANS score | Interquartile Range RBANS Score | p-value |
|---|---|---|---|---|
| Operation | CABG only | −5.5 | −17.5 – 1.5 | 0.51 |
| Involving valvular replacement | −12 | −15.3 – (−1.3) | ||
| Operation | CABG (including combination surgeries) |
−6 | −17.5 − 0.25 | 0.87 |
| Only valvular | −7 | −12.8 – 1 | ||
| Hypertension | Has diagnosis | −6 | −17 – 0 | 0.23 |
| No diagnosis | −2 | −5 – 4 | ||
| Hyperlipidemia | Has diagnosis | −5 | −14.5 – 1.5 | 0.16 |
| No diagnosis | −12 | −27 – (−4) | ||
| Diabetes | Has diagnosis | −6 | −19 – (−2) | 0.28 |
| No diagnosis | −5 | −12 – 3 | ||
| Heart Failure | Has diagnosis | −11 | −17.5 – 1 | 0.12 |
| No diagnosis | −5.5 | −16.3 – 0.25 | ||
| Smoking | Any smoking history | −6 | −18.3 – (−1.3) | 0.34 |
| Never smoked | −5 | −14 – 5.3 | ||
| Smoking | Current smoker | −2 | −19 – 0.5 | 0.78 |
| Not currently smoking | −6 | −16.5 – 0.5 | ||
| Atrial Fibrillation after Surgery | Had Atrial Fibrillation | −5 | −13 – 0.25 | 0.76 |
| No Atrial Fibrillation | −6 | −16 – (−2) | ||
| Not used post-op | −9.5 | −17 – (−2) | ||
| Intra-operative Transfusion | Transfused | −10 | −13 – 2 | 0.99 |
| Not transfused | −6 | −17 – 0 | ||
| Peri-operative Transfusion | Transfused | −13 | −31.3 – (−3.8) | 0.12 |
| Not transfused | −4.5 | −12.8 – 0.8 | ||
| Allogenic Transfusion Throughout Hospitalization | Transfused | −10 | −25.5 – (−2) | 0.17 |
| Not transfused | −4 | −14 – 0.5 | ||
| Allogenic or Autologous Transfusion Throughout Hospitalization | Allogenic or Autologous Transfusion |
−6 | −18.5 – (−1.5) | 0.17 |
| Transfusion Naive | −4 | −12 – 4.5 | ||
| Autologous Transfusion | Autologous Transfusion | −5.5 | −17.8 – (−0.5) | 0.36 |
| Transfusion Naive | −4 | −12 – 4.5 | ||
| Allogenic Transfusion | Allogenic Transfusion | −10 | −25.5 – (−2) | 0.12 |
| Transfusion Naive | −4 | −12 – 4.5 |
Correlations of dichotomous demographic and surgery characteristics of study participants. Each category split the study cohort into two groups described in the second column. The median and interquartile range of the difference in RBANS standardized scores from baseline to POD4 of patients in the different groups are listed in the third and fourth column. The last column is the p-value of the unpaired Mann-Whitney U test comparing the difference in RBANS standardized scores from baseline to POD4 between the two patient groups.
Table 3:
Correlations of Demographic and Surgical Characteristics with Neurocognitive Decline Continuous Variables
| Demographics and Surgery Characteristics | Median | Interquartile Range for Characteristic | R value | p-value |
|---|---|---|---|---|
| Age (years) | 68.5 | 64.3 – 73 | −0.02 | 0.94 |
| Morning of the operation Systolic BP (mmHg) | 139 | 124 – 149 | −0.01 | 0.97 |
| Operative time (minutes) | 185 | 159.8 – 265.3 | −0.21 | 0.26 |
| Time on Bypass (minutes) | 87 | 65 – 124 | −0.11 | 0.56 |
| Cross-clamp Time (minutes) | 63 | 48 – 97 | −0.19 | 0.33 |
| ICU Length of Stay (days) | 3 | 2 – 4 | −0.35 | 0.06 |
| Hospital Length of Stay (days) | 7 | 6 – 9 | −0.45 | 0.01* |
| Lowest Patient Temperature on Bypass (°C) | 35.4 | 34.6 – 35.9 | −0.04 | 0.82 |
| Lowest MAP on Bypass (mmHg) | 59.5 | 56 – 63 | −0.35 | 0.07 |
| Nadir Hematocrit During Bypass (%) | 22.5 | 20.8 – 24.5 | 0.32 | 0.09 |
| Platelets prior to surgery (x10^9/L) | 189.5 | 175.3 – 239.3 | −0.31 | 0.10 |
| Albumin prior to surgery | 4.15 | 3.65 – 4.4 | 0.08 | 0.70 |
| Hematocrit prior to surgery (%) | 40.9 | 36.7 – 44.4 | 0.39 | 0.03* |
| Hematocrit 6 hours after surgery (%) | 32.0 | 29.9 – 34.4 | 0.20 | 0.29 |
| Hematocrit POD4 (%) | 27.2 | 23.7 – 29.5 | 0.33 | 0.08 |
| Change in Hematocrit from Baseline to POD4 (%) |
−14.4 | −17.7 – (−12.1) | −0.20 | 0.30 |
Correlations of continuous variables both prior, during and after surgery. The studied demographic and surgery characteristics are in the first column. The median and interquartile ranges of the variables among the study participants are in the second and third column. The correlation between the variable and the difference in RBANS standardized scores from baseline to POD4 were conducted using two-tailed spearman test. The r and p values are reported in the fourth and fifth columns.
Significance is indicated with and defined as p < 0.05.
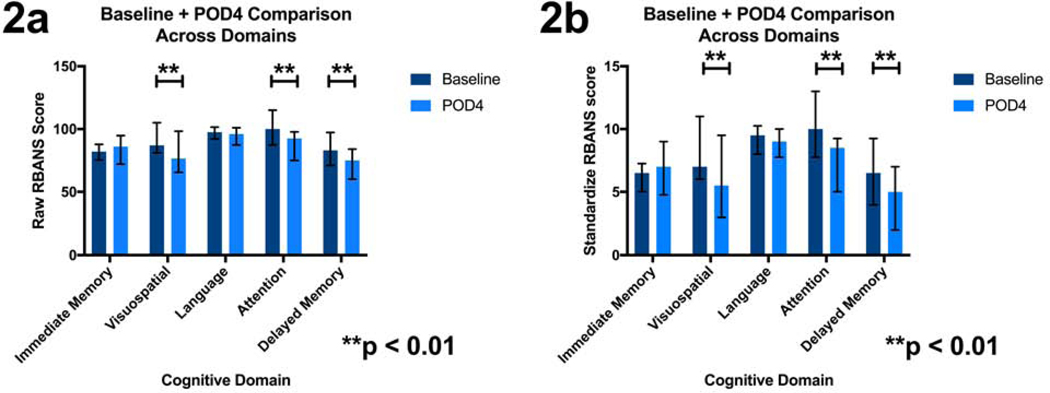
When examining the raw individual cognitive domain score, visuospatial (p=0.004), attention (p<0.0001), and delayed memory (p<0.0001) had significant differences between baseline and POD4 (Figure 2a, Table 4). The scaled individual scores also demonstrated significant differences in visuospatial (p=0.0043), attention (p<0.0001) and delayed memory (p<0.0003) between baseline and POD4 (Figure 2b, Table 4).
Figure 2.
Figure 2a: Patients’ RBANS cognitive domain raw scores compared at baseline and POD4. 2b: Standardized RBANS cognitive domain scores compared at baseline and POD4. Median and interquartile range are marked. Paired Wilcoxon rank test was used to compare group. An * denotes significance and is defined as p < 0.05, and ** is defined as p < 0.01.
Table 4:
Change in Individual Cognitive Domain from Baseline to POD4
| Raw scores | Scaled score | |||||
|---|---|---|---|---|---|---|
| Cognitive Domain | Median | Interquartile Range | p-value | Median | Interquartile Range | p-value |
| Immediate Memory Baseline POD#4 |
82 | 76 – 87 | 0.76 | 6.5 | 5 – 7 | 0.43 |
| 86 | 73.75 – 93 | 7 | 5 – 8.75 | |||
| Visuospatial Baseline POD#4 |
87 | 81 – 105 | 0.004** | 7 | 6 – 11 | 0.0043** |
| 76.5 | 66.75 – 95 | 5.5 | 3.25 – 8.75 | |||
| Language Baseline POD#4 |
97.5 | 92 – 101 | 0.29 | 9.5 | 8 – 10 | 0.27 |
| 96 | 88.5 – 101 | 9 | 8 – 10 | |||
| Attention Baseline POD#4 |
100 | 88 – 113.5 | <0.0001** | 10 | 8 – 12.75 | <0.0001** |
| 92.5 | 76 –97 | 8.5 | 5.25 – 9 | |||
| Delayed Memory Baseline POD#4 |
83 | 72–.25 | <0.0001** | 6.5 | 4.25 – 9 | 0.0003** |
| 75 | 61 – 83.25 | 5 | 2.25 – 6.75 | |||
This table looks at the change among individual cognitive domains tested with RBANS from baseline to POD4. The cognitive domains define the rows, while the type of score: raw or scaled defines the columns. Within each cognitive domain, there is a report of the median and interquartile ranges for that domain at baseline and on POD4; as well as the p-value for the paired Wilcoxon rank test comparing the scores in that domain at baseline and POD4.
Significance is indicated with if p < 0.05
if p < 0.01.
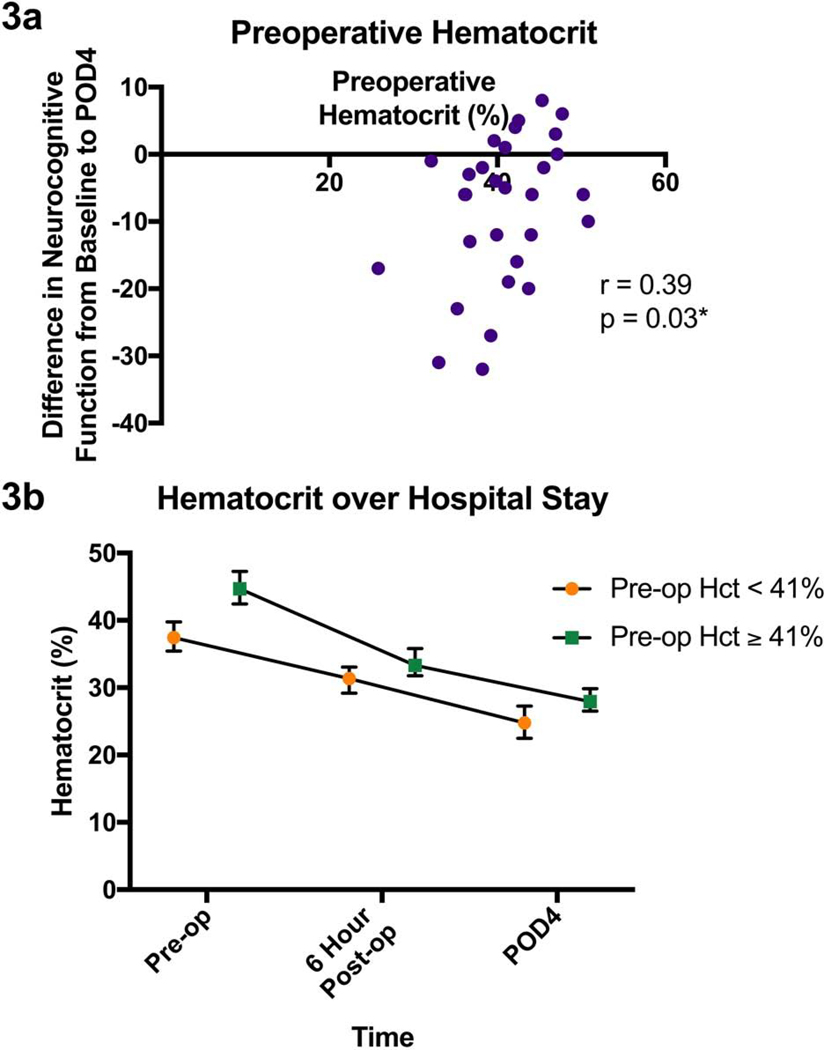
Hematocrit pre-operatively was found to be associated with worsened neurocognitive functioning on POD4 (r=0.39, p=0.03, Figure 3a). There was a trend toward hematocrit < 40% being associated with NCD (p = 0.0536, U = 65 on the Mann-Whitney U test), but there was no significant threshold that was correlated with NCD. Other markers pre-operatively were not associated with this decrease in neurocognitive function. The hematocrit six hours after the operation (r=0.20, p=0.29) did not correlated with an increased NCD on POD4, while hematocrit on POD4 showed a trend toward NCD (r=0.33, p=0.08). All patients’ hematocrit decreased over the course of their hospital stay, and patients with a lower hematocrit prior to the operation tended to have a lower hematocrit throughout their hospital stay (Figure 3b). The population is split around the hematocrit value of 41% in the figure, as that is the lower end of normal. The change in hematocrit from pre-operative values to POD4 were not significantly associated with NCD (r= −0.20, p=0.30).
Figure 3.
Figure 3a: Patients’ hematocrit levels vs their change in standardized neurocognitive scores from baseline to POD4. R and p values are also shown for the corresponding spearman two-tailed test. 3b: The trajectory of hematocrit values throughout patients’ hospital stay: prior to surgery, 6 hours after surgery and on post-op day 4. The patients are split into two groups based on their hematocrit prior to surgery. Those with a hematocrit less than 41% prior to surgery had consistently lower hematocrit levels at other time points represented here. Median and interquartile range are shown. Significance is indicated with an * and defined as p < 0.05.
Effect of blood transfusion
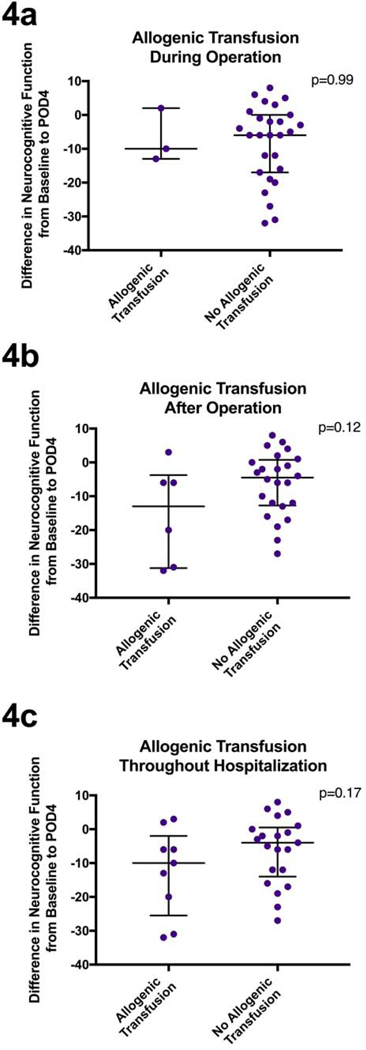
Thirty percent of patients in our study received an allogenic transfusion of packed red blood cells at some time during their hospitalization. Twenty percent of all the patients studied received the transfusion after surgery. Among those with a hematocrit below 41% preoperatively 5 out of 16 (32%) received a transfusion, 4 out of 14 (29%) received a transfusion among those with hematocrit great than 41% preoperatively.
Patients who received a transfusion during the operation (p=0.99, Figure 4a, Table 2) did not demonstrate a significant decrease in neurocognitive function. Looking specifically at patients who received a transfusion after the operation (p=0.12, Figure 4b, Table 2) or at any point during their hospitalization (p=0.17, Figure 4c, Table 2), there was a trend towards a greater NCD on POD4 in those who received the transfusion as compared to those that did not.
Figure 4.
Figure 4a: Patients’ change in standardized neurocognitive scores from baseline to POD4 compared between those that received an allogenic transfusion during the operation and those that did not. 4b: Patients’ change in standardized neurocognitive scores from baseline to POD4 compared between those that received an allogenic transfusion after their operation and those that did not. 4c: Patients’ change in standardized neurocognitive scores from baseline to POD4 compared between those that received an allogenic transfusion at any point in their hospitalization and those that did not. Analyzed using unpaired Mann-Whitney U test. Median and interquartile ranges are represented.
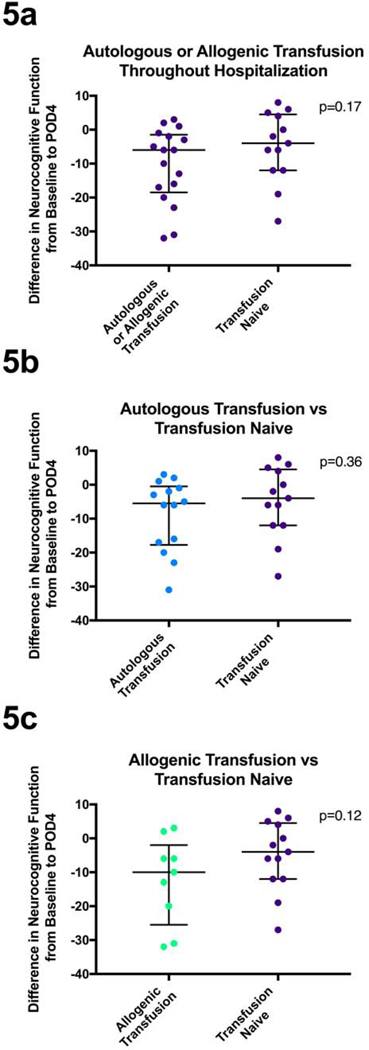
Though transfusion discussions usually exclusively consider allogenic transfusion, autologous transfusion has become a common practice. Forty-seven percent of patients in our study received an autologous transfusion during their operation. Specifically, these patients would have one or two units of blood removed in the operating room, prior to the incision, and have the blood transfused back to them at the completion of procedure. Collectively, patients who received either an autologous or allogenic transfusion did not have a significant change in their neurocognitive function on POD4 as compared to those who did not receive either – the transfusion naïve group (p=0.17, Figure 5a, Table 2). There was, however, a trend toward greater NCD in the transfusion group. We then compared each type of transfusion against the transfusion naïve group. Patients who received an autologous transfusion as compared to patients who did not receive any blood during their hospitalization, had no significant difference in their change in neurocognitive function on POD4 (p=0.36, Figure 5b, Table 2). The comparison between allogenic transfused patients and those who never received blood during their hospitalization showed a trend (p=0.12, Figure 5c) toward those transfused being associated with a greater NCD on POD4.
Figure 5.
Figure 5a: Patients’ change in standardized neurocognitive scores from baseline to POD4 compared between those that received an allogenic or autologous transfusion at any point in their hospitalization and those that did not. 5b: Patients’ change in standardized neurocognitive scores from baseline to POD4 compared between those that received an autologous transfusion and those did not receive any blood products. 5c: Patients’ change in standardized neurocognitive scores from baseline to POD4 compared between those that received an allogenic transfusion and those did not receive any blood products. Analyzed using unpaired Mann-Whitney U test. Median and interquartile ranges are represented.
Discussion
In this study we found that a lower hematocrit prior to cardiac surgery was associated with a greater incidence of early neurocognitive decline. The lack of any standard laboratory values in the six hour post-operative or POD4 time correlating with a decrease in neurocognitive function supports the existing evidence that no demographic or standard laboratory characteristics are able to predict those individuals who will develop NCD.3 However, the significant correlation of a lower hematocrit prior to surgery correlating with NCD is a new finding. Predisposing factors to NCD have been a focus of recent studies, with one being able to predict the development of NCD after cardiac surgery based on gene expression of inflammatory cytokines.7 A limiting factor in this study is that no causal relationship between hematocrit and NCD was determined, nor was a mechanism examined. The lower hematocrit could be viewed as a modifiable risk factor that could be improved prior to surgery with transfusion or could be a reflection of more chronically ill patients, who have lower hematocrit at baseline and therefore having worse outcomes with cardiac surgery. However, none of the comorbidities examined in our study: hypertension, hyperlipidemia, diabetes, or smoking history were found to predispose patients to worse cognitive outcomes on POD4. Though we did not examine the mechanism of this effect, other studies examining the relationship between mortality and other outcomes after cardiac surgery have failed to determine a clear mechanism, and it is in all likelihood multifactorial.3
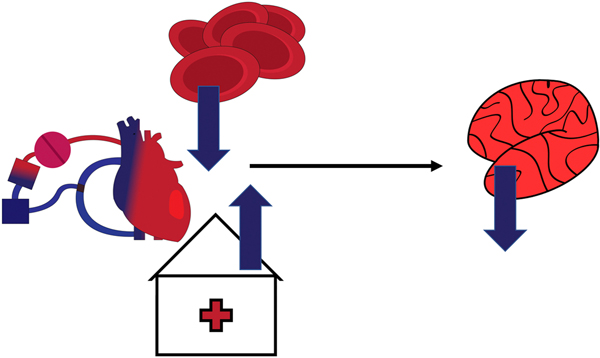
Interestingly, we demonstrated that a prolonged hospital stay was correlated with decreased cognitive function on POD4. Patients with a prolonged hospitalization could have been in more critical condition on POD4 contributing to their worse cognitive results. These findings may be expected as the total hospital length of stay is an indicator of critically ill patients with multiple factors that may contribute to NCD, or a complicated post-surgical course. Both the association of longer hospitalization and lower hematocrit prior to cardiac surgery being correlated with a decrease in cognitive function are captured in Figure 6.
Figure 6.
This is a representative image of the findings of this paper. Moving left to right, there is a representation of the heart on the cardiopulmonary bypass machine. Above and to the right are several red blood cells with an arrow going down to represent decreasing hematocrit. Below is a building with a cross with an arrow going up to represent prolonged hospitalization. To the right following the arrow is a picture of a brain with an arrow going down representing neurocognitive decline. This figure captures the findings of our study that a low hematocrit, and prolonged hospitalization in the setting of cardiopulmonary bypass are associated with neurocognitive decline.
The 1999 ACC/ACA Guideline for CABG surgery separated post-surgical NCD into two types. Type 1 is described as “a major focal neurological deficit, stupor, or coma,” and type 2 as “deterioration of intellectual function.”8 Due to the lack of focal deficits seen in type 2 neurocognitive decline, without a thorough investigation of cognitive function before and after surgery, medical professionals are not able to identify this deterioration. While risks for post-surgical neurocognitive dysfunction are multifactorial, many are non-modifiable, such as age and level of education. However, neither the pathophysiology nor any modifiable risk factors have been identified that can prevent this undesired consequence of CPB.3 These clinical findings have been replicated in a prospective, interventional study of rats after cardiopulmonary bypass, where those rats that underwent cardiopulmonary bypass surgery had impaired memory and learning deficits both one and six weeks after surgery.9 It is interesting that among our cohort of patients the visuospatial, attention and delayed memory were most affected after surgery.
A proposed mechanism of the cognitive decline is increased inflammatory cytokines during and after surgery leading to axonal CNS injury.3 In fact, the increased expression of inflammatory markers such as C-reactive protein, cytokines, and activated complement have been established as a postoperative marker for NCD.3,8
The unique finding in our study was that a lower hematocrit pre-operatively was associated with greater NCD, while a trend was present between hematocrit on POD 4 and NCD. This was independent of any other blood laboratory values. Of note, hematocrit is not an ideal marker of red blood cells in the blood, as it represents red cell mass with respect to total blood volume, and thus is influenced by the state of hydration. However, it is universally used in medicine to assess blood volume and a patient’s need of a red blood cell transfusion.
Hematocrit decreased in all patients throughout their hospitalization. This can be explained by hemodilution, bleeding and hemolysis during surgery. There is evidence that hemolysis continues after surgery due to the mechanical stress on blood cells after coming in contact with the non-endothelial surface of the cardiopulmonary bypass circuit and the state of inflammation in patients after surgery.10,11,12 Perhaps having a lower hematocrit prior to surgery is a marker of chronic illness making certain patients more susceptible to this injury. Recovery of the bone marrow with time and packed red blood cell transfusion are the only mechanisms that can increase the red blood cell quantity, therefore increasing measured hemoglobin and hematocrit in post-surgical patients.
The current study was not powered to find a statistically significant association between either intraoperative or peri-operative allogenic transfusions and neurocognitive decline. However, a trend was present. Those that received allogenic transfusions were more likely to develop NCD on POD4 as compared to both patients that did not receive allogenic transfusions and those that never received any blood, including autologous transfusions. We studied patients at relatively low risk for bleeding and transfusion; thus, the rate of transfusion was low in our cohort of patients. This trend is not explained by those having a low hematocrit prior to surgery, as rates of transfusion were similar among groups with higher and lower hematocrits before surgery.
We, as many cardiac surgical programs, have instituted a program to minimize bleeding and blood transfusion. In our hospital, the practice of pre-banking blood from the patients prior to the start of the operation and reinfusing at the end of the case is a common practice. Pre-banking blood prior to surgery has been found to be associated with higher platelet counts and hematocrit after surgery.8 The practice of pre-banking blood has been associated with decreased need for allogenic blood transfusions and decreased transfusion related complications.13,14 However, there is a concern that some patients may not be able to attain a high enough hematocrit through compensatory erythropoiesis prior to surgery.15 This risk did not appear to significantly affect the patients in our study as they were not more likely to develop NCD as compared to their non-transfused counterparts.
Historically, patients undergoing cardiac surgical procedures were readily transfused if their hemoglobin fell below, what is now known as “the liberal threshold” of 9–10 g per deciliter, to prevent the harmful consequences of anemia.16 A lower hematocrit theoretically could lead to a reduction in tissue oxygenation, leading to ischemia. In cardiac surgical patients, the cardiopulmonary bypass circuit causes additional hemolysis, which releases free hemoglobin that binds nitric oxide, decreasing nitric oxide in the circulation, thus compromising microvasculature.17,18,19 In addition, free hemoglobin may activate protein kinase C.20 These have been shown to cause vasoconstriction in various vascular beds and lead to tissue ischemia including renal injury.21
Though initially viewed as a benign treatment, transfusion was discovered to cause “systemic inflammatory response, induces nonspecific immunosuppression and perhaps occludes local microvasculature causing local tissue hypoxemia.”22 It additionally can cause harm as a result of a clerical error, blood incompatibility, alloimmunization, sepsis, immunosuppression, viral transmission, and transfusion associated lung injury.8,23,24 Furthermore, in a randomized control trial of patients admitted to intensive care units in 25 hospitals in Canada, patients were randomized to either receive a transfusion with a hemoglobin concentration below 7 g per deciliter or below 10 g per deciliter. It was found that the mortality was higher in patients who received more transfusions.16 However, a recent trial of over five thousand cardiac surgery patients randomized to receive a transfusion at a liberal or restrictive threshold found that the restrictive threshold was not inferior in terms of all-cause mortality, myocardial infarction, stroke or new-onset renal failure than its liberal threshold counterpart. This relationship was present both at the one and six months follow up. Their liberal threshold was defined as 9.5 g per deciliter in the operation and intensive care unit, and as 8.5 g per deciliter in the non-intensive care unit setting; whereas their restrictive threshold was defined as 7.5 g per deciliter in all settings.25,26 These studies suggest that a lower threshold does not incur the risk of transfusion, while having no additional mortality or morbidity in the form of ischemic changes.
It is interesting that both anemia and transfusion seem to be associated with a decline in neurocognitive function after cardiac surgery. Thus, it is difficult at this point in time to make conclusive recommendations regarding transfusion thresholds in order to optimize neurocognitive function.
Future directions
It will be important to understand the mechanism of hematocrit prior to surgery and its correlation with NCD on POD4. Is the lower hematocrit a product of chronic bone marrow suppression due to chronic illness or a modifiable risk factor that with a transfusion prior to surgery could improve patients’ outcomes? It is worth exploring if the significant changes in visuospatial, attention and delayed memory functioning on POD4 correlate with structural and functional changes in areas of the brain responsible for these actions using a functional magnetic resonance imaging study. Understanding if the correlations and associations discovered in this study are present in a larger population will be an important next step as well. With a greater population, it will be important to conduct a multivariate analysis that we were unable to conduct in this study due to the small sample size. We would also aim to follow the patients further out after surgery in order to determine if the variables throughout their hospitalization correlate with long-term NCD and a reduction in quality of life.
Limitation of the study
The limitations of this study include the small size and predominantly white, male population enrolled. This is important, as there is evidence that women have a greater drop in their hemoglobin after cardiac surgery mainly due to preexisting anemia and greater hemodilution.27 Due to the multiple testing conducted in this study, there is also risk of a type 1 error and that these findings were due to chance, rather than a clinically significant correlation. There is a selection bias of less ill patients enrolling in our study, as study participants needed to be capable of consenting to the study preoperatively, not have any of the conditions that were among the exclusion criteria and capable of completing the initial RBANS assessment. Finally, no mechanism was determined or examined for the findings in our study. However, this seems to be the case for all prior similar studies.
Conclusion
In this study we found that a lower hematocrit prior to cardiac surgery was associated with a greater incidence of early neurocognitive decline. Studies have examined the risk of a moderately low hematocrit during cardiac surgery and found that it does not lead to increased mortality or easily measured morbidity events. Our study examines the effect low hematocrit has on a difficult to measure adverse event – neurocognitive decline. A lower hematocrit correlates with cognitive decline, perhaps necessitating a reexamination of clinical practice.
Acknowledgements:
Funding for this research was provided by the National Heart, Lung, and Blood Institute [R01HL46716; R01HL128831 (F.W.S)]; NIH/NIGMS Training Grant T32 GM065085 (B.A.P. and L.A.S.) and T34HL09430809 (N.C.S.). NIH T35 HL094308 training grant to A.Y.G.
Funding/Support: None of the authors have any financial disclosures.
Glossary of Terms
- CABG
Coronary artery bypass graft
- CPB
Cardiopulmonary bypass
- HCT
Hematocrit
- ICU
Intensive Care Unit
- NCD
Neurocognitive decline
- POD4
= Post-operative day 4
- RBANS
Repeatable Battery of Assessment of Neuropsychological Status
Footnotes
COI/Disclosure: None of the authors have any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moazzami K, Dolmatova E, Maher J, et al. In-Hospital Outcomes and Complications of Coronary Artery Bypass Grafting in the United States Between 2008 and 2012. J Cardiothorac Vasc Anesth. 2017;31(1):19–25. doi: 10.1053/j.jvca.2016.08.008 [DOI] [PubMed] [Google Scholar]
- 2.Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal Assessment of Neurocognitive Function after Coronary-Artery Bypass Surgery. N Engl J Med. 2001;344(6):395–402. doi: 10.1056/NEJM200102083440601 [DOI] [PubMed] [Google Scholar]
- 3.Ramlawi B, Rudolph JL, Mieno S, et al. C-Reactive protein and inflammatory response associated to neurocognitive decline following cardiac surgery. Surgery. 2006;140(2):221–226. doi: 10.1016/j.surg.2006.03.007 [DOI] [PubMed] [Google Scholar]
- 4.Kastaun S, Gerriets T, Schwarz NP, et al. The Relevance of Postoperative Cognitive Decline in Daily Living: Results of a 1-Year Follow-up. J Cardiothorac Vasc Anesth. 2016;30(2):297–303. doi: 10.1053/j.jvca.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 5.Wolfe R, Worrall-Carter L, Foister K, Keks N, Howe V. Assessment of Cognitive Function in Heart Failure Patients. Eur J Cardiovasc Nurs. 2006;5(2):158–164. doi: 10.1016/j.ejcnurse.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 6.Randolph C Repeatable Battery for the Assessment of Neuropsychological Status Update. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 7.Sabe AA, Dalal RS, Chu LM, et al. Preoperative gene expression may be associated with neurocognitive decline after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2015;149(2):613–623. doi: 10.1016/j.jtcvs.2014.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA Guidelines for Coronary Artery Bypass Graft Surgery: Executive Summary and Recommendations. Circulation. 1999;100(13):1464–1480. [DOI] [PubMed] [Google Scholar]
- 9.Homi HM, Calvi CL, Lynch J, Grocott HP. Longitudinal Assessment of Neurocognitive Function in Rats After Cardiopulmonary Bypass: Evidence for Long-Term Deficits. J Cardiothorac Vasc Anesth. 2010;24(2):293–299. doi: 10.1053/j.jvca.2009.07.020 [DOI] [PubMed] [Google Scholar]
- 10.Cirri S, Negri L, Babbini M, et al. Haemolysis due to active venous drainage during cardiopulmonary bypass: comparison of two different techniques. Perfusion. 2001;16(4):313–318. doi: 10.1177/026765910101600408 [DOI] [PubMed] [Google Scholar]
- 11.Kameneva MV, Undar A, Antaki J, Watach M, Calhoon JS, Borovetz H. Decrease in Red Blood Cell Deformability Caused by Hypothermia, Hemodilution, and Mechanical Stress. Vol 45; 1999. doi: 10.1097/00002480-199907000-00010 [DOI] [PubMed] [Google Scholar]
- 12.Ricci Z, Pezzella C, Romagnoli S, et al. High levels of free haemoglobin in neonates and infants undergoing surgery on cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2014;19(2):183–187. 10.1093/icvts/ivu129. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich W, Thuermel K, Heyde S, Busley R, Berger K. Autologous Blood Donation in Cardiac Surgery: Reduction of Allogeneic Blood Transfusion and Cost-Effectiveness. J Cardiothorac Vasc Anesth. 2005;19(5):589–596. doi: 10.1053/j.jvca.2005.04.017 [DOI] [PubMed] [Google Scholar]
- 14.Britton LW, Eastlund DT, Dziuban SW, et al. Predonated autologous blood use in elective cardiac surgery. Ann Thorac Surg. 1989;47(4):529–532. doi: 10.1016/0003-4975(89)90427-X [DOI] [PubMed] [Google Scholar]
- 15.Kasper SM, Gerlich W, Buzello W. Preoperative red cell production in patients undergoing weekly autologous blood donation. Transfusion. 1997;37(10):1058–1062. doi: 10.1046/j.1537-2995.1997.371098016445.x [DOI] [PubMed] [Google Scholar]
- 16.Hébert PC, Wells G, Blajchman MA, et al. A Multicenter, Randomized, Controlled Clinical Trial of Transfusion Requirements in Critical Care. N Engl J Med. 1999;340(6):409–417. doi: 10.1056/NEJM199902113400601 [DOI] [PubMed] [Google Scholar]
- 17.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 18.Vaughn MW, Huang K-T, Kuo L, Liao JC. Erythrocytes Possess an Intrinsic Barrier to Nitric Oxide Consumption. J Biol Chem. 2000;275(4):2342–2348. doi: 10.1074/jbc.275.4.2342 [DOI] [PubMed] [Google Scholar]
- 19.Liao JC W Hein T, Vaughn MW, Huang K-T, Kuo L. Intravascular flow decreases erythrocyte consumption of nitric oxide. Proc Natl Acad Sci. 1999;96(15):8757 LP–8761. http://www.pnas.org/content/96/15/8757.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishizawa S, Nezu N, Uemura K. Direct evidence for a key role of protein kinase C in the development of vasospasm after subarachnoid hemorrhage. J Neurosurg. 1992;76(4):635–639. doi: 10.3171/jns.1992.76.4.0635 [DOI] [PubMed] [Google Scholar]
- 21.Minneci PC, Deans KJ, Zhi H, et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115(12):3409–3417. doi: 10.1172/JCI25040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Surgenor SD, DeFoe GR, Fillinger MP, et al. Intraoperative Red Blood Cell Transfusion During Coronary Artery Bypass Graft Surgery Increases the Risk of Postoperative Low- Output Heart Failure. Circulation. 2006;114(1 suppl):I-43-I-48. [DOI] [PubMed] [Google Scholar]
- 23.Popovsky MA. Transfusion-related acute lung injury. Curr Opin Hematol. 2000;7(6). https://journals.lww.com/co-hematology/Fulltext/2000/11000/Transfusion_related_acute_lung_injury.14.aspx. [DOI] [PubMed] [Google Scholar]
- 24.Hart A, Khalil JA, Carli A, Huk O, Zukor D, Antoniou J. Blood Transfusion in Primary Total Hip and Knee Arthroplasty. Incidence, Risk Factors, and Thirty-Day Complication Rates. JBJS. 2014;96(23). https://journals.lww.com/jbjsjournal/Fulltext/2014/12030/Blood_Transfusion_in_Primary_Total_Hip_and_Knee.2.aspx. [DOI] [PubMed] [Google Scholar]
- 25.Mazer CD, Whitlock RP, Fergusson DA, et al. Restrictive or Liberal Red-Cell Transfusion for Cardiac Surgery. N Engl J Med. 2017;377(22):2133–2144. doi: 10.1056/NEJMoa1711818 [DOI] [PubMed] [Google Scholar]
- 26.Mazer CD, Whitlock RP, Fergusson DA, et al. Six-Month Outcomes after Restrictive or Liberal Transfusion for Cardiac Surgery. N Engl J Med. August 2018. doi: 10.1056/NEJMoa1808561 [DOI] [PubMed] [Google Scholar]
- 27.Gombotz H, Schreier G, Neubauer S, Kastner P, Hofmann A. Gender disparities in red blood cell transfusion in elective surgery: a post hoc multicentre cohort study. BMJ Open. 2016;6(12). http://bmjopen.bmj.com/content/6/12/e012210.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]