Figure 4: Detection of DSBs by PIKKs and downstream DDC signaling in budding yeast.
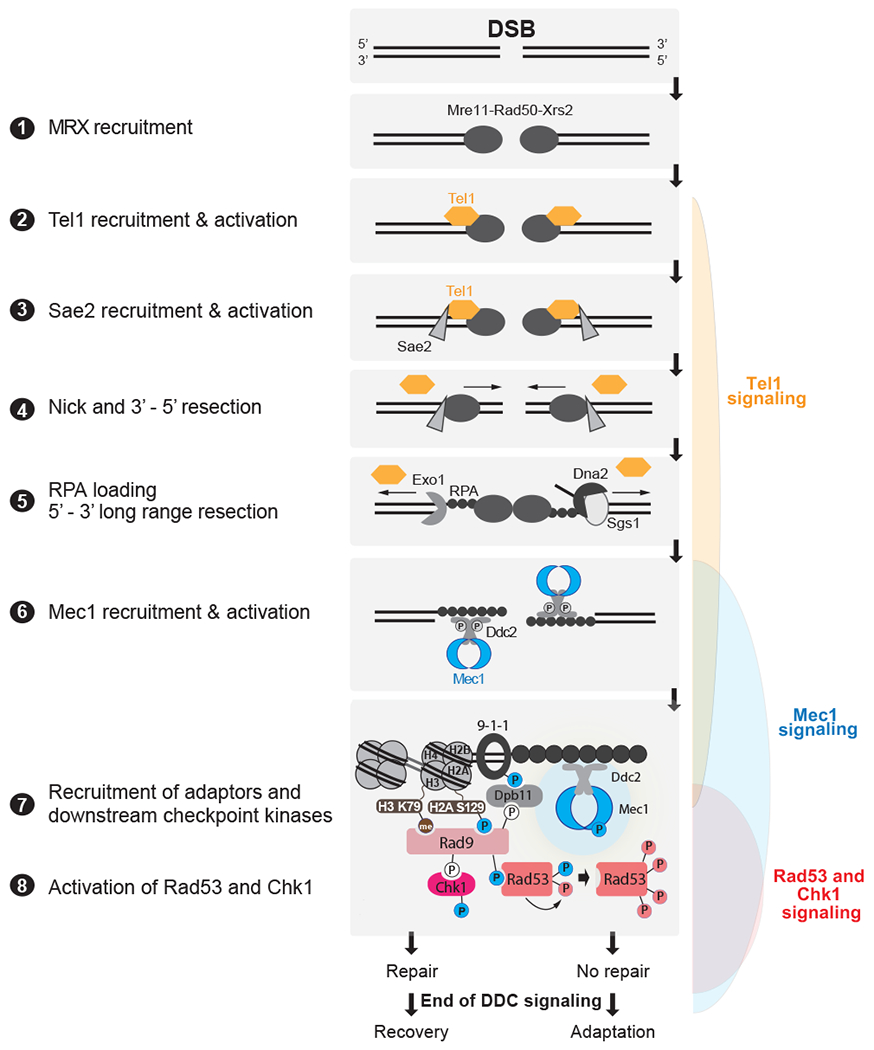
DSBs are first recognized by the MRX complex, which binds to the broken ends. MRX recruits the PIK kinase Tel1 and Sae2 to begin DSB end processing by Exo1 and Dna2. RPA coats ssDNA, leading to the recruitment of Mec1-Ddc2 dimers. The 9-1-1 clamp loader assembles the 9-1-1 clamp at the 5’ recessed end of the dsDNA/ssDNA junction. Dpb11 is recruited to 9-1-1 via a Mec1-dependent phosphorylation in the Ddc1 subunit. Mec1 and Tel1 phosphorylate histone H2A on S129 (Y-H2AX). Rad9 bound to Chkl is recruited to Y-H2AX through Rad9’s BRCT domain and histone H3K79me through Rad9’s TUDOR domain. Rad9 is shown here as monomeric for simplicity. Mec1 then phosphorylates and activates Chk1 and phosphorylates Rad9, priming Rad9 for Rad53 recruitment. Rad53 binds phosphorylated Rad9 allowing for Mec1-dependent phosphorylation and activation. Activated Rad53 phosphorylates and activates other Rad53 molecules leading to full checkpoint activation. Tel1 in yeast contributes a very modest role to activating Rad53 and Chk1. Bottom most panel shows key interactions and phosphorylation events involved in activation of the DDC. Color of phosphorylation sites (circles with “P”) refer to the kinase responsible: blue=Mec1, red=Rad53, white=CDK, gray=unknown. See text for additional details.
