Introduction
In December 2019 a novel coronavirus, SARS-CoV-2, was identified as the pathogen causing coronavirus disease 2019 (COVID-19) in Wuhan, Hubei Province, China.1 By the end of January 2020, the World Health Organization declared the outbreak of SARS-CoV-2 a Public Health Emergency of International Concern.2 Respiratory illness remains the main clinical manifestation of COVID-19, but involvement of other systems, including the cardiovascular system, has been well documented. It is estimated that up to 19.7% of patients were noted to have cardiac injury, based on literature from Wuhan, China.3,4 Cardiac arrhythmia has been reported in 16.7% of patients, without further specification.2 Effects on the conduction system have not been reported, besides a few case reports.5,6 We present a case series of 3 patients with otherwise normal cardiac structure and function and without prior cardiac history who were admitted to the intensive care unit with COVID-19 and developed periods of complete heart block during their clinical course without any other identifiable etiology. We believe this is a novel effect of COVID-19, and thus it is important for clinicians to be aware of this potential manifestation of disease.
Case report
Case 1
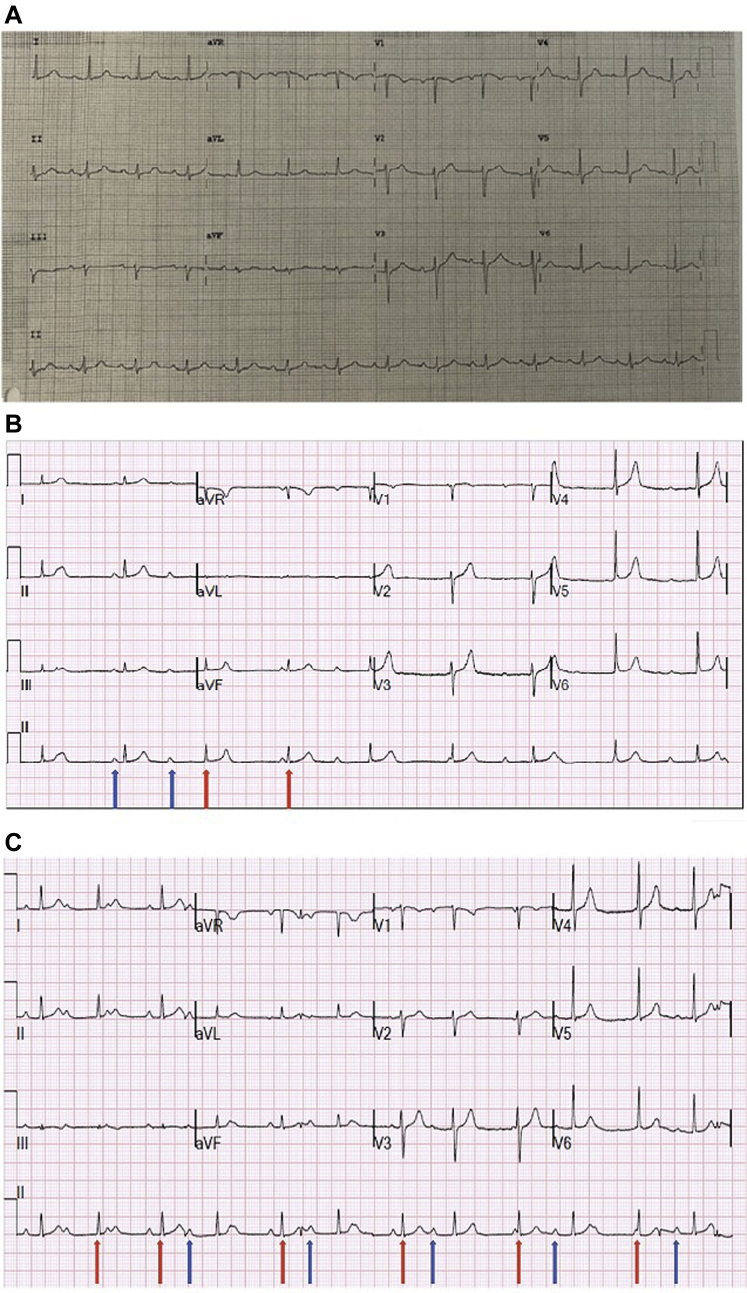
Patient 1 is an 82-year-old man with a medical history significant for hypertension and prior stroke who presented to the emergency department complaining of shortness of breath and a dry cough for 4 days. On arrival to the emergency department, he was noted to be in severe hypoxic respiratory distress requiring immediate endotracheal intubation. Chest radiography revealed patchy bilateral parenchymal opacities. Clinical presentation was highly suspicious of COVID-19. A nasopharyngeal swab test for COVID-19 on real-time reverse transcriptase polymerase chain reaction assay was positive. His initial lab work-up was remarkable for lymphopenia (779 cells/mm3), elevated BUN/Cr (22.7), mild transaminitis (AST 115 U/L and ALT 83 U/L), electrolytes all within normal limits, and elevated inflammatory markers (CRP 12.27 mg/dL, procalcitonin 0.37 ng/mL, fibrinogen 757 mg/dL, and D-dimer 2.91 μg/mL FEU); creatinine phosphokinase was elevated (796 U/L), but troponin T was normal (<0.01 ng/mL). Electrocardiogram (ECG) on presentation showed normal sinus rhythm at a rate of 82 beats per minute (bpm), normal intervals, normal axis, and no ST-T wave changes (Figure 1). This was unchanged from prior outpatient ECGs.
Figure 1.
A: Electrocardiogram (ECG) of patient 1 obtained on presentation demonstrating normal sinus rhythm at a rate of 82 beats per minute (bpm) with normal intervals, normal axis, and no ST-T wave changes. B: ECG of patient 1 on day 1 of admission demonstrating complete heart block with an independent atrial rate at 80 bpm (blue arrow) and narrow complex escape ventricular rhythm at a rate of 50 bpm (red arrow). C: Patient 1 on day 3 of admission demonstrates escape-capture Wenckebach with a pattern of a conducted beat with a long PR (blue arrows) followed by a dropped beat and then apparent nonconduction of the subsequent P wave owing to occurrence of a junctional escape beat (red arrows). The significant variability of the R-R intervals and association of shorter R-R intervals with P waves demonstrates presence of some degree of AV conduction.
Shortly after endotracheal intubation and on stable ventilator settings, the patient was noted to be in complete heart block on telemetry. Figure 2A shows an electrocardiogram performed at this time. Minimal change in heart rate was noted and there were no pauses. Further work-up included normal thyroid function, serial negative cardiac biomarkers, and normal cardiac function on echocardiogram. Review of medication list was remarkable for absence of atrioventricular (AV) node blocking agents. He was sedated with propofol and dexmedetomidine, which were both discontinued, and yet the patient continued to manifest with heart block with a narrow escape rhythm with stable hemodynamics for 6 days (Figure 2B). These ECGs showed intermittent Wenckebach pattern and escape-capture Wenckebach consistent with AV nodal disease. His respiratory illness continued to worsen, requiring proning on multiple occasions to improve his oxygenation.
Figure 2.
Rhythm strip obtained on day 6 of admission for patient 2 and demonstrating likely atrioventricular nodal location of block with 2:1 conduction, as evidenced by progressive prolongation of PR interval (blue arrows) followed by nonconducted P waves (red arrow) and 2:1 block.
His clinical course was further complicated by catheter-associated bloodstream infection with Staphylococcus epidermidis. Unfortunately, his hypoxic respiratory failure continued to worsen and his inflammatory markers remained elevated. His family eventually elected to focus on comfort measures and the patient died on hospital day 16.
Case 2
Patient 2 is a 55-year-old man with no significant medical history who presented to the emergency department with a dry cough and a sore throat for 1 week. A nasopharyngeal swab was positive for COVID-19 and the patient was sent home to self-quarantine. He presented 6 days later with progressive shortness of breath. His initial vitals were significant for a fever of 101.1°F, tachypnea, and an oxygen saturation of 85%. He developed persistent hypoxemia despite oxygen therapy, requiring intubation. Initial laboratory work-up revealed elevated inflammatory markers (ESR 90 mm/h, procalcitonin 0.41 ng/mL, D-dimer 2.74 μg/mL FEU, fibrinogen 736 mg/dL, LDH of 666 U/L, and CRP 22.9 mg/dL). His other laboratory work-up was within normal limits, including a normal troponin T. His initial ECG showed a heart rate of 90 bpm with a right bundle branch block and as well as ST depressions in the inferior leads that were present at his baseline. Chest radiography revealed bilateral ground-glass opacities. He completed a 5-day course of hydroxychloroquine and azithromycin for COVID-19.
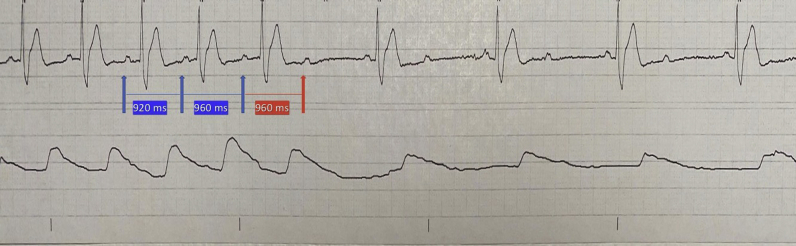
His course was complicated by shock on day 2 of admission, requiring vasopressor support. He progressed into multiorgan failure and developed acute kidney injury and hepatic failure as well as elevated troponin level (peak troponin T level was 1.35 ng/mL). Owing to the sudden change in clinical status, a bedside echocardiogram was done, which revealed right heart strain and McConnell’s sign concerning for pulmonary embolism. The patient received tissue plasminogen activator, with improvement in hemodynamic status. He was initiated on continuous renal replacement therapy for his worsening kidney function. A repeat echocardiogram 1 day after tissue plasminogen activator administration revealed improved right ventricular function.
On day 6 of admission, the patient was noted to have bradycardia. Telemetry revealed a second-degree AV block with 2:1 conduction (Figure 3A). These episodes of AV block lasted for less than a minute but recurred repeatedly. His electrolytes were checked at that time and were all within normal limits. The patient continued to have 2:1 block for 3 days intermittently despite removal of all sedation. At that time the patient was not on any AV nodal blocking agents.
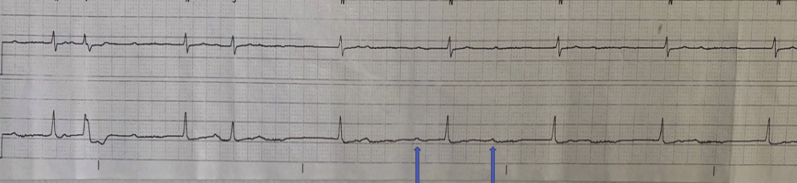
Figure 3.
Rhythm strip for patient 3 demonstrating complete atrioventricular (AV) block with AV dissociation, as evidenced by independent atrial rate of 60 beats per minute (bpm) (blue arrow) and ventricular rate of 40 bpm.
AV block resolved and the patient was successfully extubated on day 15 and was discharged to a long-term acute care hospital.
Case 3
Patient 3 is a 43-year-old man with no significant medical history who initially presented with shortness of breath, fever, myalgias, and diarrhea for 5 days. On arrival to the emergency department, the patient was noted to be febrile with a temperature of 101°F, tachycardic with a heart rate of 105 bpm, and hypoxemic with an oxygen saturation of 88% on room air. Chest radiography revealed bilateral infiltrates. Initial lab work-up revealed mildly elevated white blood cell count with absolute lymphocytes (750 cells/mm3), elevated procalcitonin (1.41 ng/mL), LDH (458 U/L), and normal troponin T <0.01 ng/mL. ECG on admission showed normal sinus rhythm at 74 bpm, normal intervals, normal axis, and absence of ischemic findings.
His course was complicated by progressive hypoxemia despite maximal oxygen therapy, requiring endotracheal intubation, paralysis, and proning. On day 6, his nasopharyngeal swab tested positive for SARS-CoV-2 by polymerase chain reaction. He completed a 5-day course of hydroxychloroquine and azithromycin. His course was further complicated by a superimposed bacterial infection requiring broad-spectrum antibiotics.
On day 22 of admission another repeat test for SARS-CoV-2 was still positive. On day 24 of admission, the patient was noted to be in intermittent complete heart block on telemetry for less than 24 hours (Figure 3B). His inflammatory markers at that time remained elevated, including CRP (34.4 mg/dL), LDH (318 U/L), ferritin (1237 ng/mL), fibrinogen (954 mg/dL), and D-dimer (3.8 μg/mL FEU); electrolytes were all within normal limits. Echocardiogram was unremarkable, with normal ejection fraction and no effusion. The patient received a tracheostomy owing to prolonged intubation and was discharged to a long-term acute care facility.
Discussion
Disease of the AV node happens occasionally in severely ill patients.7 Possible mechanisms for transient impairment include metabolic alterations, enhanced vagal tone owing to pain, sleep, carotid sinus manipulation during usual nursing and caring of patients, or medication side effects. These multifactorial mechanisms tend to lead to intermittent and often first- or second-degree AV block. Higher-grade AV block as described previously in our clinical vignettes suggests another pathology of AV conduction disturbance.
Underlying etiologies for new-onset AV node disease include ischemic cardiac disease, thyroid disease, anatomical disturbances, medications, and infections.8,9 In our case series, neither troponin elevation nor ventricular dysfunction was associated with AV block, suggesting either focused involvement of the AV node or a secondary cause of AV block associated with the critical illness and organ dysfunction. The nature of AV block in these cases suggests the origin of block is in the AV node. The evidence for this is that the escape rhythm tended to be narrow, similar to sinus in morphology, and of adequate rate to support normal hemodynamics without requiring temporary pacing. In addition, 2 patients showed evidence of a Wenckebach pattern recorded during onset or resolution of AV block, providing further evidence that the location of block was in the AV node, and therefore suggesting a benign prognosis of the AV block.
Similar involvement of the conduction system has been previously described with other infectious etiologies, including but not limited to Lyme carditis. Steere and colleagues10 suggested a predilection for AV node involvement in Lyme carditis, proximal to the bundle of His. Further electrophysiologic studies from case reports support this pattern of involvement.11,12 The mechanism of AV nodal block is believed to be owing to an inflammatory process secondary to direct invasion of heart tissue by Borrelia burgdorferi with almost complete resolution of AV block in up to 6 weeks.13,14
There have been many speculations on cardiovascular disease manifestations of SARS-CoV-2 infection. Possible etiologies for cardiac manifestations involve hypoxemia, electrolyte abnormalities, microthrombi, inflammatory surge from the cytokine storm, and/or direct invasion of the cardiomyocytes. Inflammatory cells and SARS-CoV-2 virus have been identified in the myocardium in autopsy studies, suggesting direct invasion of the heart in some cases.15 The implication of development of AV block during critical illness with SARS-CoV-2 infection remains unknown. Whether this will have permanent effects remains questionable, but the resolution of AV block in all patients in this series without the need for pacemaker placement suggests that these patients may not suffer long-term ill effects.
It is also notable that we have observed several cases of severe sinus bradycardia and sinus node dysfunction associated with SARS-CoV-2 infection. We did not include these cases in this series, as sinus bradycardia may be a relatively common finding in patients who are intubated and treated with sedatives. However, there may be an association if extremely high vagal tone is postulated as the mechanism for AV block in these cases.
We believe it is pertinent for clinicians to be aware of such worrisome potential effect of SARS-CoV-2 infection, and thus our aim in presenting this case series is to create awareness of a possible effect of COVID-19 on the conduction system and encourage future investigations and studies to further characterize the potential effects of this virus on the cardiac conduction system.
Key Teaching Points.
-
•
COVID-19 can potentially manifest with atrioventricular (AV) block. The nature of AV block as seen in these cases suggests the origin of block is in the AV node. The evidence for this is that the escape rhythm tended to be narrow, similar to sinus in morphology, and of adequate rate to support normal hemodynamics.
-
•
Whether AV node involvement in COVID-19 will have permanent effects remains unknown, but the resolution of AV block in these patients without the need for pacemaker placement suggests that they may not suffer long-term ill effects.
-
•
Future investigations and studies are needed to further characterize the potential effects of SARS-CoV-2 on the cardiac conduction system.
Footnotes
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
References
- 1.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assaad IE, Hood-Pishchany MI, Kheir J, et al. Complete heart block, severe ventricular dysfunction and myocardial inflammation in a child with COVID-19 infection. JACC Case Rep. https://doi.org/10.1016/j.jaccas.2020.05.030. 2020.05.27. [DOI] [PMC free article] [PubMed]
- 6.Azarkish M., Laleh Far V., Eslami M., Mollazadeh R. Transient complete heart block in a patient with critical COVID-19. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa307. ehaa307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Artucio H., Pereira M. Cardiac arrhythmias in critically ill patients: epidemiologic study. Crit Care Med. 1990;18:1383–1388. doi: 10.1097/00003246-199012000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Zoob M., Smith K.S. Aetiology of complete heart-block. Br Med J. 1963;2:1149–1154. doi: 10.1136/bmj.2.5366.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lev M. Anatomic basis for atrioventricular block. Am J Med. 1964;37:742–748. doi: 10.1016/0002-9343(64)90022-1. [DOI] [PubMed] [Google Scholar]
- 10.Steere A.C., Batsford W.P., Weinberg M. Lyme carditis: cardiac abnormalities of Lyme disease. Ann Intern Med. 1980;93:8–16. doi: 10.7326/0003-4819-93-1-8. [DOI] [PubMed] [Google Scholar]
- 11.Reznick J.W., Braunstein D.B., Walsh R.L. Lyme carditis. Electrophysiologic and histopathologic study. Am J Med. 1986;81:923–927. doi: 10.1016/0002-9343(86)90370-0. [DOI] [PubMed] [Google Scholar]
- 12.van der Linde M.R., Crijns H.J., Lie K.I. Transient complete AV block in Lyme disease. Electrophysiologic observations. Chest. 1989;96:219–221. doi: 10.1378/chest.96.1.219. [DOI] [PubMed] [Google Scholar]
- 13.McAlister H.F., Klementowicz P.T., Andrews C., Fisher J.D., Feld M., Furman S. Lyme carditis: an important cause of reversible heart block. Ann Intern Med. 1989;110:339–345. doi: 10.7326/0003-4819-110-5-339. [DOI] [PubMed] [Google Scholar]
- 14.Haddad F.A., Nadelman R.B. Lyme disease and the heart. Front Biosci. 2003;8:s769–s782. doi: 10.2741/1065. [DOI] [PubMed] [Google Scholar]
- 15.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14:247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]