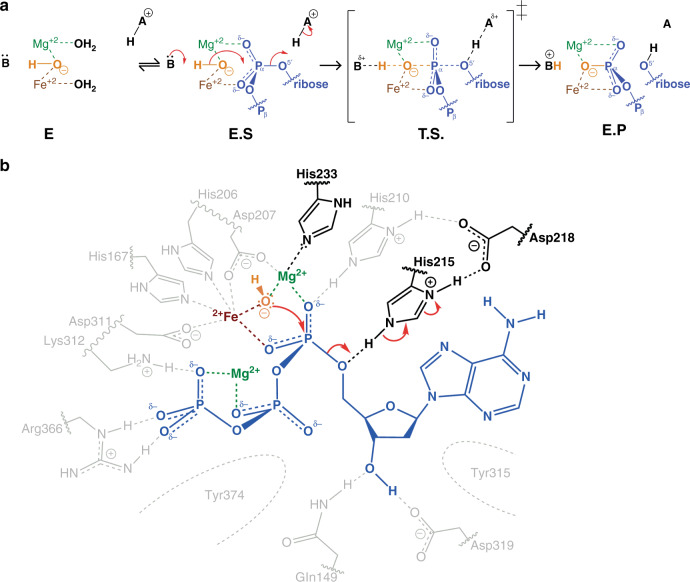
Fig. 5. Catalytic mechanism of SAMHD1-dNTP hydrolysis.
a Schematic of the chemical mechanism of SAMHD1-dNTP hydrolysis. In the apo state (E) the W0 water molecule (orange) is coordinated between the HD motif-bound Fe ion and by Mg3, further water molecules take up the remaining coordination positions on the metal ions. On substrate binding, the enzyme–substrate complex (E.S) is formed and Pα oxygens replace water molecules to coordinate the Mg3 and Fe ions and also position the W0 nucleophile in line with the electron deficient α-phosphate. The reaction proceeds by adduction of the W0 nucleophile to the α-phosphate to form a trigonal-bipyramidal intermediate transition state (T.S.). The resulting accumulating negative charge is relieved by protonation of the leaving nucleoside 5′ oxygen to form the enzyme product complex (E.P). b Schematic of SAMHD1-active site conformation during the hydrolysis reaction. A bound substrate dATP nucleotide is shown in blue, W0 in orange, the HD Fe ion in brown and active site Mg ions in green. The side chains of His233 and His215 and Asp218 required for catalysis are highlighted and the direction of electron transfer is indicated by the red arrows.