Abstract
Outbreaks of trichinellosis caused by Trichinella papuae have been reported in South-East Asia. Mebendazole and thiabendazole are the treatments of choice for trichinellosis; however, both drugs result in significant side effects and are less effective for muscle-stage larvae (L1). An alternative therapeutic agent is needed to improve treatment. Information on lipid composition and metabolic pathways may bridge gaps in our knowledge and lead to new antiparasitics. The T. papuae L1 lipidome was analysed using a mass spectrometry-based approach, and 403 lipid components were identified. Eight lipid classes were found and glycerophospholipids were dominant, corresponding to 63% of total lipids, of which the glycerolipid DG (20:1[11Z]/22:4[7Z,10Z,13Z,16Z]/0:0) (iso2) was the most abundant. Overall, 57% of T. papuae lipids were absent in humans; therefore, lipid metabolism may be dissimilar in the two species. Proteins involved T. papuae lipid metabolism were explored using bioinformatics. We found that 4-hydroxybutyrate coenzyme A transferase, uncharacterized protein (A0A0V1MCB5) and ML-domain-containing protein are not present in humans. T. papuae glycerophospholipid metabolic and phosphatidylinositol dephosphorylation processes contain several proteins that are dissimilar to those in humans. These findings provide insights into T. papuae lipid composition and metabolism, which may facilitate the development of novel trichinellosis treatments.
Subject terms: Chemical biology, Lipidomics
Introduction
Trichinella is a genus of parasitic roundworms that cause trichinosis, also known as trichinellosis, which infect domestic and sylvatic animals. The number of global outbreaks appears to have sharply increased, reflecting changes in the parasites’ epidemiology1. In South-East Asia, outbreaks of trichinellosis caused by T. papuae occurred in 20062 and 20073. T. papuae, which belongs to the non-encapsulated trichinella clade, lives predominantly in tropical rainforests4. Ingestion of raw meat containing parasite cysts leads to infection, and the larvae released from adult females invade host muscles resulting in trichinellosis pathology. Common symptoms are eye puffiness, splinter haemorrhaging, nonspecific gastroenteritis, and muscle pain5. Trichinosis treatment is based on anti-inflammatory drugs and anthelmintics, such as mebendazole and albendazole6; however, the effectiveness of anthelmintic treatment is an issue of debate. In the treatment of myositis during a trichinellosis outbreak in Thailand, mebendazole and thiabendazole were found to be more efficient than placebo or fluconazole. However, 30% of volunteers could not tolerate the side effects of thiabendazole7. In addition, in a trial during an outbreak in Italy, between 3% and 45% of patients had a recurrence of various symptoms after a 10-day mebendazole course8. Generally, anthelmintic therapy is only considered effective during the intestinal phase of infection, and the drug has poor drug effectiveness in the muscle phase9. To improve the effectiveness of the treatment, an alternative therapeutic agent may need to be developed. Most likely, a combination of two or more drugs with different modes of action will be needed for an adequate cure rate and to delay the development of parasite resistance.
Recently, high-throughput technologies have allowed characterization of the lipid profiles of parasites, including unicellular protists and worms. Specific lipid structures and their metabolic pathways could be targets for the development of novel anthelmintics. In addition, the discovery of a vital enzyme in a metabolic pathway that is absent or significantly different from that in the host is an advantage for novel target identification and drug development10. Separation techniques, such as gas chromatography (GC) and liquid chromatography (LC), are coupled with mass spectrometry (MS) for lipid detection and quantification11. The lipid profiles of leishmania12, Toxoplasma gondii13 and Haemonchus contortus14 are examples of successful lipidomic approaches. Non-mammalian lipids have been reported in parasite lipidomes, such as glycerolipids or sphingolipids terminated by α-Gal(1 → 6)β-Gal, which is unique in apicomplexans15. Information on lipid composition and lipid pathways has led to several antiparasitic designs. Glycosylphosphatidylinositols of T. gondii and Plasmodium falciparum were found to be candidates for immunotherapeutic strategies16,17. Another phosphatidylcholine metabolism in plasmodium was demonstrated to be a novel drug target18. Amphotericin B is an antiparasitic drug which binds to ergosterol, which is present in leishmania membranes and absent in mammals. The amphotericin B-induced membrane pore is responsible for ion leakage, contributing to parasite death19. Miltefosine20, edelfosine21 and sitamaquine22 are lipid-like drugs used for leishmaniasis treatment; they affect membrane lipid rafts and accumulate in L. donovani through passive diffusion.
In this research, we aimed to profile the lipid components of T. papuae larvae using MS-based lipidomics. In addition, proteins relating to lipid metabolism in T. papuae were compared with those in humans to explore potential new drug targets. The study was designed to identify drug target candidates and their related pathways.
Results
Lipid profile of T. papuae larvae
Lipid components were extracted from T. papuae L1 and analysed by liquid chromatography (LC) coupled with tandem MS (LC-MS/MS). To increase the number of lipids identified, both positive and negative electrospray ionization (ESI) modes were used for MS analysis. A total of 403 lipid components were identified from larval extracts. The positive and negative modes detected 300 and 104 lipid species, respectively (Supplementary Dataset 1). Only a polyketide, ovaliflavanone A, was found by both ionization modes, as shown in Supplementary Fig. 1. Whole lipid components of T. papuae L1 were classified into eight classes: glycerophospholipids, glycerolipids, sphingolipids, fatty acyls, sterol lipids, polyketides, prenol lipids and saccharolipids. Glycerophospholipids were the largest component, corresponding to 63% of the total lipids. While glycerolipids and sphingolipids comprised 15% and 8%, respectively. The minor lipid classes were fatty acyl lipids (6%), sterol lipids (3%), polyketides (3%), prenol lipids (1%) and saccharolipids (1%), as presented in Fig. 1. T. papuae glycerophospholipids contain fatty acids with carbon chain lengths of 6, 8,12, 13, 15, 16, 17 18, 20, 22, 24 and 26, and 79% of glycerophospholipids contained unsaturated fatty acids. T. papuae glycerolipids were found to have fatty acids with carbon chain lengths of 9, 12, 13, 14, 15, 16, 17, 18, 20, 22, 23, 24, 25 and 26, and the majority (87%) also contained unsaturated fatty acids. The sphingolipids were composed of fatty acids with carbon chain lengths of 14, 15, 16, 17, 18, 19, 20, 22, 24 and 26, and 79% of them contained unsaturated fatty acids. Because the glycerophospholipids, glycerolipids, sphingolipids and fatty acyl lipids were the major classes in T. papuae, they were further subclassified, as described in Table 1. In the glycerophospholipids, the major lipid subclass was glycerophosphocholine (PC) representing 96 species. While glycerophosphoethanolamine (PE), glycerophosphoserine (PS), glycerophosphoinositol (PI), glycerophosphoglycerol (PG) and glycerophosphate represented 75, 32, 15, 14 and 12 species, respectively. In the glycerolipids, diradylglycerol (DG) was the main subclass, with 27 species. Triradylglycerolipid (TG), digalactosyldiacylglycerol (DGDG), monoradylglycerolipid (MG) and monogalactosyldiacylglycerol (MGDG) described 23, 10, 1 and 1 species, respectively. In the sphingolipids, ceramide was the largest lipid subclass, with 17 species. Sphingomyelin, glycosphingolipid and sulphatide represented 8, 6 and 2 species, respectively. Of the fatty acyl lipids, 15 carnitines were observed, while triacontane and tetraenoic acid each described three respective species.
Figure 1.
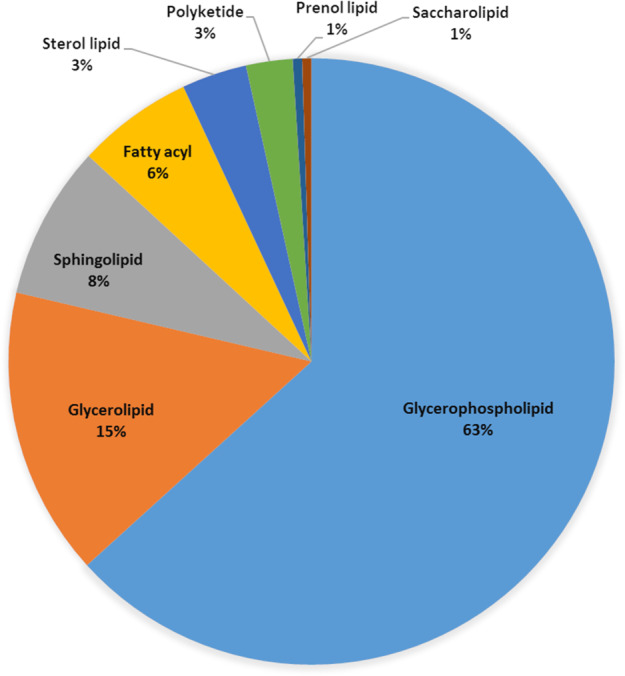
Classification of T. papuae muscle-stage larvae lipidome.
Table 1.
Subclasses of fatty acyl, glycerolipid, glycerophospholipid and sphingolipid.
| Lipid class | Subclass | No. of components |
|---|---|---|
| Fatty acyl | Carnitines | 5 |
| Triacontanes | 3 | |
| Tetraenoic acids | 3 | |
| Others | 14 | |
| Glycerolipid | Triradylglycerolipids | 23 |
| Monoradylglycerolipids | 1 | |
| Digalactosyldiacylglycerols | 10 | |
| Diradylglycerols | 27 | |
| Monogalactosyldiacylglycerols | 1 | |
| Glycerophospholipid | Glycerophosphates | 12 |
| Glycerophosphocholines | 96 | |
| Glycerophosphoethanolamines | 75 | |
| Glycerophosphoglycerols | 14 | |
| Glycerophosphoinositols | 15 | |
| Glycerophosphoserines | 32 | |
| Others | 11 | |
| Sphingolipid | Ceramides | 17 |
| Sphingomyelins | 8 | |
| Glycosphingolipids | 6 | |
| Sulphatides | 2 |
Comparison of T. papuae and human lipidomes
Each T. papuae L1 lipid component was compared to the Human Metabolome Database (HMDB). The HMDB contains the small molecule metabolites reported in humans. The metabolite data has been acquired from different experimental approaches, such as nuclear magnetic resonance (NMR) spectroscopy, gas chromatography–MS (GC-MS) and liquid chromatography–MS (LC-MS)23. In T. papuae L1, 57% of the total lipids were different from those in humans (Supplementary Dataset 1). All eight lipid classes in T. papuae were more than 50% different from human lipids. In particular, saccharolipids were 100% different between T. papuae and humans (Fig. 2).
Figure 2.
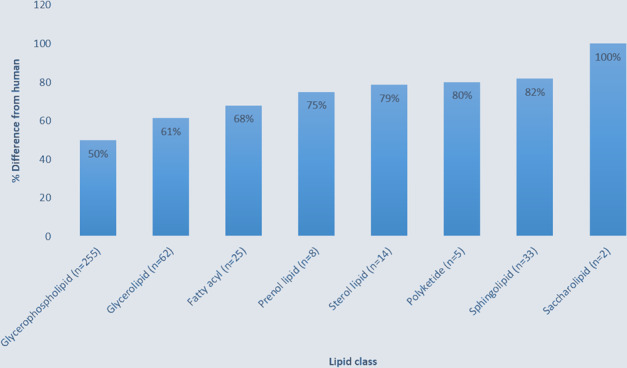
Percentage of T. papuae lipids that were different from those in humans.
Quantification of lipid components
The quantities of each lipid species can be estimated through the ion intensities of LC-MS/MS analysis. The top-10 most abundant lipids are shown in Fig. 3. A glycerolipid, DG (20:1[11Z]/22:4[7Z,10Z,13Z,16Z]/0:0) (iso2), was the most abundant lipid species in T. papuae L1. The abundance of this lipid was seven-fold greater than that of PG (21:0/20:0), the second ranked species. The glycerophospholipids, PG (21:0/20:0), PC (22:4[7Z,10Z,13Z,16Z]/16:0), PC (16:0/20:4[8Z,11Z,14Z,17Z]), PC (18:2[9Z,12Z]/18:0), PC (18:1[11Z]/16:0) and PE (18:0/22:4[7Z,10Z,13Z,16Z]), were included in the top ranking lipids after DG (20:1[11Z]/22:4[7Z,10Z,13Z,16Z]/0:0) in T. papuae L1. While, sterol lipids, e.g., alloavicholic acid and 27-norcholestanehexol, were also highly abundant. However, the total ion abundance of a specific lipid species doesn’t necessarily indicate this lipid amount since ion abundance was affected by both ionization efficiency and lipid concentration. The different lipid class has quite different ionization efficiency. Therefore, relative ion abundance of lipids from the same class were more appropriate to consider. The top-10 most abundant lipids in each class in are provided in Supplementary Table 1. The most abundant glycerophospholipids were PG (21:0/20:0), PC (22:4[7Z,10Z,13Z,16Z]/16:0) and PC (16:0/20:4[8Z,11Z,14Z,17Z]). Whereas, DG (20:1[11Z]/22:4[7Z,10Z,13Z,16Z]/0:0) (iso2), DGDG (20:2/14:1) and TG (12:0/13:0/15:1[9Z]) (iso6) were the most abundant in the glycerolipid class. The top sphingolipids were SM (d18:1/18:0), SM (d18:1/16:0) and Cer (d14:1/22:0). The fatty acyl lipids with the high yields were 4,8,16-trimethyldotriacontane, 11,21-dimethylheptatriacontane and (9Z)-3-hydroxyoctadecenoylcarnitine. With regards sterol lipids, alloavicholic acid, 27-norcholestanehexol and beta-chlorogenin were the most common. Whereas, the most abundant polyketide species were 4,5-di-O-methyl-8-prenylafzelechin-4beta-ol and ovaliflavanone A. Prenol lipids with high abundance were 35-aminobacteriohopane-32,33,34-triol, coenzyme Q10 and hemigossypol. Of the saccharolipids, butyl 4′-O-butanoyl-6-O-hexadecanoyl-neohesperidoside and DAT (18:0/22:0[2Me{S},4Me{S}]) were the most abundant species. The chemical structures of the most abundant lipids in every class were distinct from those of human lipids. This finding suggests that the lipidome of T. papuae L1 is distinct from that of Homo sapiens.
Figure 3.
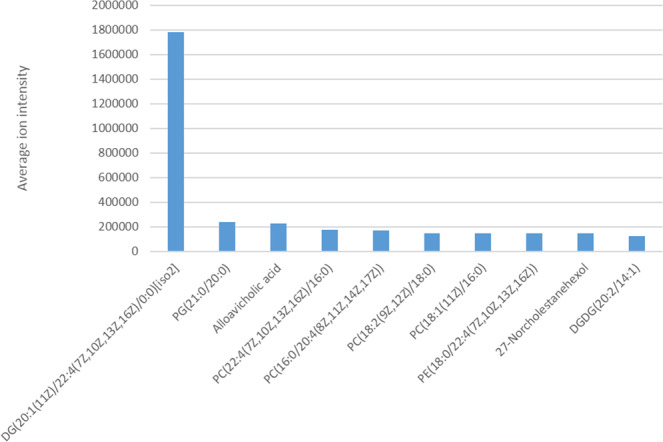
Top-10 most abundant lipids identified in T. papuae muscle-stage larvae.
T. papuae lipid mechanisms
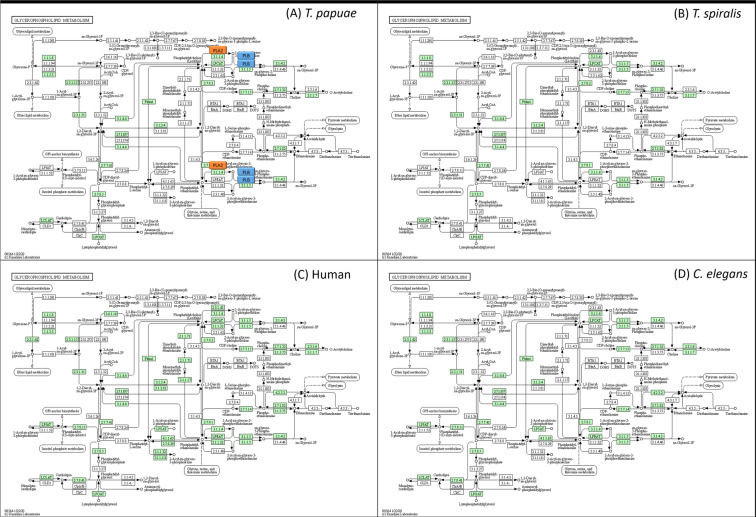
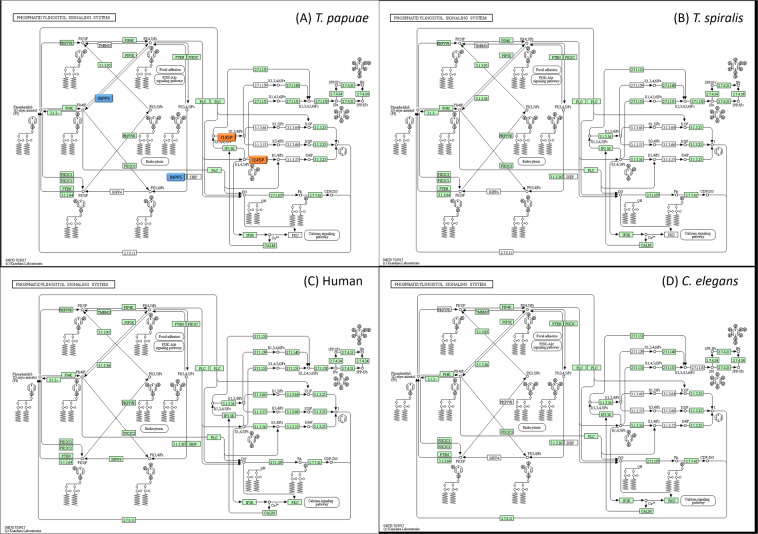
Because of the distinct structures of T. papuae L1 lipids, their metabolism and synthesis mechanisms may also differ from those of humans. To investigate the proteins involved in the lipid metabolic processes in T. papuae, a bioinformatics pipeline was designed, which is shown in Fig. 4. Multiple gene ontology (GO) can be used to annotate the function of single genes; therefore, all genes involved in T. papuae lipid metabolism were collated using QuickGO, a web-based tool for GO searching. This annotation program provides GO annotations to proteins in the UniProt Knowledgebase (UniProtKB), RNA molecules from RNACentral and protein complexes from the Complex Portal. The GO:0006629 pathway was used to search for lipid metabolism process genes and 193 gene products were found that belonged to T. papuae. All protein sequences involved in T. papuae lipid metabolic processes were subjected to the Basic Local Alignment Search Tool (BLAST) to compare their similarity to T. spiralis, T. britovi, T. native, T. pseudospiralis, C. elegans and human protein sequences. We found 41 T. papuae proteins containing less than 50% sequence similarity to human proteins (Supplementary Table 2 and Supplementary Dataset 2), providing evidence that some cellular lipid processes of T. papuae may be dissimilar to those in human. T. spiralis, T. britovi, T. native and T. pseudospiralis demonstrated high similarity to T. papuae proteins. Whereas, C. elegans protein sequences were slightly different from Trichinella species. Compared with human proteins, T. papuae 4-hydroxybutyrate coenzyme A transferase (A0A0V1M4U9), uncharacterized protein (A0A0V1MCB5) and ML domain-containing protein (A0A0V1MEM4) had the lowest sequence similarity. T. papuae 4-hydroxybutyrate coenzyme A transferase is involved in the acetate metabolic process (GO:0006083), propionate metabolic process and methylcitrate cycle (GO:0019679). Whereas, the uncharacterized protein and ML domain-containing protein are associated with the ganglioside catabolic process (GO:0006689) and sphingolipid metabolic process (GO:0006665), respectively. These three proteins are putative candidates for future anti-trichinella drug development. The entire 41 T. papuae proteins with low sequence similarity to human proteins involved in lipid metabolism were classified into the 16 lipid pathways shown in Table 2. The major pathways were the glycerophospholipid metabolic process (GO:0006650, 6 proteins), phosphatidylinositol dephosphorylation (GO:0046856, 6 proteins), acetate metabolic process (GO:0006083, 3 proteins), ganglioside catabolic process (GO:0006689, 3 proteins), glycosylphosphatidylinositol (GPI)-anchor biosynthetic process (GO:0006506, 3 proteins) and sphingolipid metabolic process (GO:0006665, 3 proteins). T. papuae glycerophospholipid metabolic and phosphatidylinositol dephosphorylation processes present the most attractive targets for drug design because they contain several proteins that are dissimilar to equivalent proteins in humans. These include phospholipase B-like (PLB) and 85/88 kDa calcium-independent phospholipase A2 (PLA2) of the glycerophospholipid metabolic process (GO:0006650, 6 proteins) (Fig. 5A) and type I inositol 1,4,5-trisphosphate 5-phosphatase (I145P), inositol polyphosphate 5-phosphatase K (INPP5) and 72 kDa inositol polyphosphate 5-phosphatase of the phosphatidylinositol dephosphorylation process (Fig. 6A). In addition, a total 15,304 protein sequences of T. papuae was collected from UniProt database and analyzed by blast2GO software to match the T. papuae proteins into the glycerophospholipid metabolism process and phosphatidylinositol dephosphorylation process as shown in Figs 5A and 6A, respectively. Moreover, the T. spiralis, C. elegans and human glycerophospholipid metabolism process and phosphatidylinositol dephosphorylation process are also demonstrated for comparison in Figs 5 and 6, respectively. The green and white boxes are proteins which present and absent in each organism. Due to these finding, proteins in both processes are similar between T. papuae and T. spiralis. They are slightly different between C. elegans and Trichinella. While, the proteins in the pathways are distinguishable between nematode and human.
Figure 4.
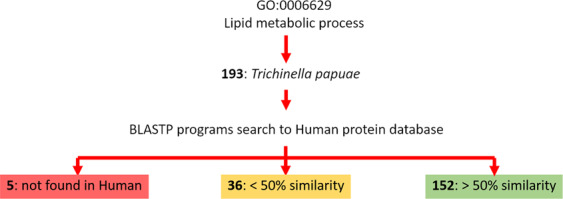
A bioinformatics pipeline for investigating proteins involved in lipid metabolic processes of T. papuae.
Table 2.
GO term classification of T. papuae proteins involved in lipid metabolic processes.
| Pathway | No. of proteins |
|---|---|
| Glycerophospholipid metabolic process [GO:0006650] | 6 |
| Phosphatidylinositol dephosphorylation [GO:0046856] | 6 |
| Acetate metabolic process [GO:0006083]; propionate metabolic process, methylcitrate cycle [GO:0019679] | 3 |
| Ganglioside catabolic process [GO:0006689] | 3 |
| GPI-anchor biosynthetic process [GO:0006506] | 3 |
| Sphingolipid metabolic process [GO:0006665] | 3 |
| Arachidonic acid secretion [GO:0050482]; lipid catabolic process [GO:0016042]; phospholipid metabolic process [GO:0006644] | 2 |
| cholesterol metabolic process [GO:0008203]; lipid catabolic process [GO:0016042] | 2 |
| fatty acid biosynthetic process [GO:0006633] | 2 |
| lipid biosynthetic process [GO:0008610] | 2 |
| lipid metabolic process [GO:0006629] | 2 |
| phosphatidylinositol-mediated signalling [GO:0048015]; phosphatidylinositol phosphorylation [GO:0046854] | 2 |
| no data | 2 |
| angiogenesis [GO:0001525]; dolichol biosynthetic process [GO:0019408] | 1 |
| farnesyl diphosphate biosynthetic process [GO:0045337]; geranyl diphosphate biosynthetic process [GO:0033384] | 1 |
| steroid biosynthetic process [GO:0006694] | 1 |
Figure 5.
Glycerophospholipid metabolic process of T. papuae (A), T. spiralis (B), human (C), C. elegans (D) The orange and blue boxes represent PLA2 and PLB, respectively. The green boxes represent proteins presenting in each organism.
Figure 6.
Phosphatidylinositol dephosphorylation process T. papuae (A), T. spiralis (B), human (C), C. elegans (D) The orange and blue boxes represent I145P and INPP5, respectively. The green boxes represent proteins presenting in each organism.
Discussion
According to our LC-MS/MS lipid analyses, both the negative and positive ESI ionization modes increased the number of identified lipids. Some sodium adducts were observed on T. papuae polar and non-polar lipids. Cation adducts form easily on the dipoles of lipids in the positive ionization mode24. In the negative mode, T. papuae PE, PI, PS, sulphatide and non-esterified free fatty acids were detected. These lipid species are acidic lipids containing ionic bonds and were simply deprotonated and ionized as negative ion species. T. papuae PC and SM were also detected in the negative ionization mode as a result of their strong zwitterionic structures which can form anionic adducts with small anions25.
In the lipid classification experiment, glycerophospholipids (also called phosphoglycerides) were identified as the major lipid class of T. papuae. Glycerophospholipids are fatty acid diglycerides with a phosphatidyl ester attached to the terminal carbon. Their abundance is likely to be related to the fact that glycerophospholipids, sphingolipids and sterols are the major constituents of membrane bilayers in eukaryotic cells, with glycerophospholipids being the most abundant26. A high percentage of glycerophospholipids has been observed in other parasites, such as Trypanosoma brucei27 and S. mansoni28. The T. papuae glycerophospholipids were sub-classified into PC (39%), PE (31%), PS (13%), PI (6%), PG (6%) and glycerophosphate (5%). Onchcerca ochengi, a filarial nematode, was found to contain PC (57.6%), PE (25.2%), PI (8.5%) and PS (7.8%)29. The glycerophospholipids of the two nematodes appear to have similar concentration trends. A study of S. mansoni glycerophospholipids reported contents of PC (28%), PE (25%), PS (15%), and PG (8%)30. The percentages of PC in T. papuae and O. ochengia are higher than that in S. mansoni, which may be related to their different body walls structures. The outer body wall of S. mansoni is a tegument of carbohydrate-containing macromolecules known as the glycocalyx31. In contrast, the body wall surface of T. papuae is coated with an extracuticular layer of lipids comprised of an abundance of PC32. PC is composed of a choline head group and glycerophosphoric acid with a variety of fatty acids, and it has roles not only in biological membranes but also in strategies by which parasitic helminths evade host immune responses. PC attached to a secreted glycoprotein ES-62 of the rodent filarial nematode Acanthocheilonema viteae is thought to be responsible for modulating host cytokine production33. PC can induce MyD88 protein expression leading to suppression of toll-like receptor 4 (TLR4) and interleukin-33 (IL-33) signalling34. Synthetic PC analogues of A. viteae reproduce many anti-inflammatory effects and have potential uses as anti-inflammatory drugs35. Furthermore, the PC components of T. papuae membranes may also play roles in host immune modulation. PE and PS contain phosphorylethanolamine and phosphoserine, respectively, at glycerol substitution sites and many different combinations of fatty acids can attach at the C-1 and C-2 positions of the glycerol. In addition, PEs and PSs are involved in cell membrane metabolism36. Oxidized PE and PS promote or suppress human inflammatory phenotypes in monocytes and myeloid dendritic cells, and they promote an inflammatory response by increasing tumour necrosis factor alpha (TNF-α) expression. However, stimulation of monocytes with oxidized PE and PS in the presence of lipopolysaccharides (LPS) results in a decrease in TNF-α and IL-1β37. Oxidized PEs and PSs have also been observed in T. papuae, and they may be involved in modulating the host inflammatory response. Furthermore, they are possible drug development candidates for human diseases related to oxidative stress and inflammation. One such glycerophospholipid class molecule in T. papuae is PI; it contains a glycerol molecule with phosphoinositide and various fatty acids and is a core component of GPI anchors. The GPI consists of PI, glycans and a terminal phosphoethanolamine linked to the C-terminus of the protein. The GPIs of Plasmodium, Trypanosoma, Leishmania and Toxoplasma are distinguishable from mammalian GPIs38. The GPI-anchoring surface antigens are involved in the pathogenicity of parasitic diseases by causing inflammatory responses and other symptoms, for example hypoglycaemia, acidosis and anaemia39. GPI-based vaccines have been demonstrated to reduce parasitaemia and malaria severity40. In addition, GPI-related pathways have been previously studied for antiparasitic purposes, e.g., for immune therapeutics and novel drug design41–43. There are differences in the structural details of PIs between T. papuae and humans, such as oxidization and fatty acid composition. Therefore, T. papuae PIs and related biological mechanisms might be useful areas for anti-trichinella research.
With regards to glycerolipids, their structures are composed of a glycerol condensed with one, two, or three fatty acids, yielding MG, DG and TG, respectively. In most eukaryotes, glycerolipids are mainly involved in lipid storage. In Toxoplasma, TG is de-novo synthesized through an acyl‐CoA:diacylglycerol acyltransferase‐mediated pathway localized to the parasite endoplasmic reticulum44. DGAT (acyl-CoA: diacylglycerol acyltransferase) is a transmembrane enzyme that is thought be the rate-limiting enzyme for glycolipid synthesis. DGAT also presents in T. papuae as a hypothetical protein T10_12081 (GenBank: KRZ77819.1). The T. papuae protein showed 72% sequence similarity to the diacylglycerol O-acyltransferase of Brachionus plicatilis (data not shown). For that reason, T. papuae may synthesize glycerolipids through a similar pathway to B. plicatilis, and T. papuae DGAT may be a good candidate for drug development. Galactolipids, such as MGDG and DGDG, are also members of the glycerolipid class. Interestingly, the MGDGs and DGDGs identified in T. papuae were completely distinct from the human galactolipids. DGDGs in P. falciparum and T. gondii are also different from those in humans, but they show similar properties to plant DGDGs analysed by chromatographic lipid separation45. A thorough examination of the galactolipid biosynthesis pathway of T. papuae may provide further insights into lipid metabolism in this parasite.
The backbone of sphingolipid is a sphingoid (long-chain) base, which is usually linked to a fatty acid via an amide bond. Sphingolipids are important for cell division, differentiation, cell death, and cell recognition and signalling, and sphingolipid biosynthesis pathways have been proposed as promising targets for therapeutic intervention46. A key step in the synthesis of the complex sphingolipids is the glucosylation of ceramide, which is mediated by ceramide glucosyltransferase (CGT). CGT of C. elegans has been reported to have roles in oocyte formation and early embryonic cell division47. A CGT inhibitor, 1-phenyl-2-palmitoylamino-3-morpholino-1-propanol, was able to reduce cyst transformation of Giardia lamblia by 90%48. In T. papuae, CGT also presents as a ceramide glucosyltransferase-B (GenBank: KRZ80132.1), and it could represent a drug target for trichinellosis treatment. The schistosomal sphingolipids induce T helper 2 cell responses in helminth infections through T cell stimulation49. A structural analogue of sphingosine, 2-amino-2[2-(4-octylphenyl)ethyl]-1,3-propanediol (fingolimod), is a new generation of immunomodulator for several autoimmune diseases and has recently completed a phase II clinical trial for sclerosis50. Sphingolipids may be involved in T. papuae cellular processes and may also act as anti-inflammatory molecules during host immune evasion.
Sterols are necessary for cell membrane structure and function and act as precursors for several steroid hormones. Sterols are generated from acetyl-CoA via a multistep metabolic pathway that includes the key steps acetoacetyl-CoA thiolase, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) synthase and HMG-CoA reductase. These three enzymes are expressed in T. papuae as 3-ketoacyl-CoA thiolase (GenBank: KRZ75105.1), hydroxymethylglutaryl-CoA synthase 1 (GenBank: KRZ69053.1) and HMG-CoA reductase (GenBank: KRZ76401.1). Statin is one of the main classes of sterol biosynthesis inhibitors, which inhibit HMG-CoA reductase and have been widely used for cholesterol reduction in humans51. In T. cruzi, inhibitors of HMG-CoA reductase, such as mevinolin (lovastatin), reduced the growth rate of amastigotes. Furthermore, a combination of mevinolin, terbinafine and ketoconazole showed a synergistic effect on amastigotes52. Therefore, interference of the T. papuae sterol biosynthesis pathway is a potential approach for anti-trichinella development.
Neutral-lipid and glycolipid uptake have been observed in parasitic nematodes, and, although the actual mechanism of lipid absorption has not been investigated, it is likely to be a form of diffusion53. Because of the chemical properties of lipids, they must be transported through aqueous environments by other molecules. There are two main types of lipid binding proteins (LBPs) found in nematodes; one carries lipids through aqueous compartments and the other transports lipids across cell membranes. LBPs may also be involved in cellular functions, nutrient acquisition, host–parasite interactions and pathogenesis54. Long-chain fatty acid transport protein 1 (GenBank: KRZ68592.1) and fatty acid-binding-like protein 6 (GenBank: KRZ72130.1) were found in T. papuae and may play roles in lipid uptake or transportation. In addition, lipid metabolic processes are important for parasitic nematodes. In our study, three T. papuae lipid metabolic proteins, 4-hydroxybutyrate coenzyme A transferase, uncharacterized protein (A0A0V1MCB5) and ML domain-containing protein, are conserved among Trichinella species and not found in humans. In T. papuae, 4-hydroxybutyrate coenzyme A transferase takes part in the fermentation of 4-aminobutyrate to ammonia, acetate and butyrate and has been found in anaerobic bacteria such as Clostridium aminobutyricum and Porphyromonas gingivalis55. T. papuae juveniles within nurse cells use facultative anaerobic metabolism56, thus, they may use this enzyme for the same purpose as anaerobic bacteria. There is no published information on uncharacterized protein (A0A0V1MCB5) and ML domain-containing protein in T. papuae. Therefore, it would be useful to characterize these proteins to understand their functions, as they might be potential drug targets for further anti-trichinella development.
The glycerophospholipid metabolic and phosphatidylinositol dephosphorylation processes could be adopted as pathways for drug design because they contain several proteins that are dissimilar to the equivalent proteins in humans. Two phospholipases, PLB and PLA2, are involved in glycerophospholipid metabolism. Three phosphatases, I145P, INPP5 and 72 kDa inositol polyphosphate 5-phosphatase, play roles in the T. papuae phosphatidylinositol dephosphorylation process. PLB has structural features similar to Ntn-hydrolases and it could catalyze the hydrolysis of specific amide bonds of many substrates such as antibiotics and proteins57. The PLB contributes to the virulence of many pathogens, for example Candida albicans, Cryptococcus neoformans, Aspergillus spp., Legionella pneumophila and Pseudomonas aeruginosa, through host membrane lysis58,59,. When triazole, a synthetic phospholipase inhibitor, was administered to mice infected with C. albicans, parasite tissue penetration was blocked and host death prevented60. In case of PLA2, it is an important energy source enzyme and crucial in the development of Clonorchis sinensis mediated hepatic fibrosis61. PLA2 has been reported its role on membrane remodelling and vesicle secretion in several organisms for example P. falciparum62 and Leishmania amazonensis63. Plasmodial PLA2 involves the egress of merozoites from liver-stage schizonts64 and merozoite invasion of red blood cells58. According to phospholipase roles in other organisms, T. papuae phospholipases are potential targets for further drug development. I145P, INPP5 and 72 kDa inositol polyphosphate 5-phosphatase are related to secondary-messenger-mediating cell responses to various stimulations and regulate Golgi-vesicular trafficking65. No studies have been carried out on T. papuae I145P, INPP5 or 72 kDa inositol polyphosphate 5-phosphatase in term of their roles and functions. Therefore, it would be useful to characterize these proteins for a better understanding of T. papuae lipid metabolic processes.
Our lipidomics study of T. papuae larvae provided insights into the lipids and lipid metabolism in the parasites, revealing several potential drug targets which may facilitate the development of innovative parasite control mechanisms. The glycerophospholipid metabolism process and phosphatidylinositol dephosphorylation process are different between T. papuae and human. However, the lipidome of T. spiralis, an encapsulated Trichinella species, will need to be studied and comparatively analysed with that of T. papuae. These studies may lead to the development of effective prevention and control strategies against trichinosis caused by both Trichinella clades.
Methods
T. papuae preparation
The T. papuae used in this study were laboratory strains maintained in the Department of Helminthology, Faculty of Tropical Medicine, Mahidol University, Thailand. Six-week-old female ICR mice were orally infected with 100 larvae. After 2 months of infection, muscle stage larvae (L1) were obtained from the muscle tissue by pepsin digestion (0.7% pepsin [BDH, UK], 0.7% HCl). All procedures performed on animals in this study were approved by the Faculty of Tropical Medicine Animal Care and Use Committee (FTM-ACUC), Mahidol University. The approval number was FTM-ACUC No. 009/2555. All experiments were performed in accordance with relevant guidelines and regulations
Lipid extraction
T. papuae L1 were quickly frozen in liquid nitrogen and then finely ground in a pestle and mortar. Liquid/liquid extraction was then performed following the Bligh and Dyer method66. Briefly, the lipids were extracted from the finely ground L1 by adding 1:1 (v/v) chloroform/methanol and mixed using a vortex. The mixture was then centrifuged at 3,000 × g for 10 min to achieve phase separation. The lower chloroform phase was carefully transferred to a separate 1.5-ml tube and dried in a fume hood.
Ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS)
Lipid profile analysis was conducted using ACQUITY UPLC with a 2777 C autosampler (Waters Corp, USA) coupled to a Xevo G2-XS QTof mass spectrometer (Waters, Manchester, UK) with an ESI source67. For each sample, triplicate injections were made for each ionization mode. Intact molecular masses and associated fragmentation products were recorded for all compounds using MS, during which retention-time aligned data were collected in two channels simultaneously, i.e., low collision energy for precursor ions and high collision energy for daughter ions, with no precursor ion selection in the quadrupole. A mass range of m/z 100 to 2000 was acquired for lipids. High mass accuracy was maintained by lock mass correction using leucine enkephalin as a reference mass (m/z 556.2771 in ESI+ and m/z 554.2615 in ESI-).
For lipid profiling, the total lipid extracts were solubilized in 2:1:1 (v/v/v) isopropanol/acetonitrile/water (40 µl) and vortex mixed until complete dissolution. The solution was then diluted with mobile phase A at 1:20 dilution, vortex mixed and transferred to a LC-MS sample vial. The samples were kept at 4 °C in the autosampler, 3 µl was injected into the column, and the samples were separated using an ACQUITY UPLC CSH C18 column (2.1 × 100 mm, 1.7 µm; Waters) with a column temperature of 55 °C. Mobile phase A was composed of 60:40 (v/v) acetonitrile/water with 10 mM ammonium formate and 0.1% formic acid. Mobile phase B was composed of 90:10 (v/v) isopropanol/acetonitrile with 10 mM ammonium formate and 0.1% formic acid. The elution gradient was set as follows: 40–43% B (0.0–2.0 min), 43–50% B (2.0–2.1 min), 50–54% B (2.1–12.0 min), 54–70% B (12.0–12.1 min), 70–99% B (12.1–18.0 min), 99–44% B (18.0–18.1 min) and 40% B (18.1–20 min) with a flow rate 400 µl/min. For the ionization source, capillary voltages of 2.0 kV and 1.0 kV were employed for ESI + and ESI-, respectively, and a 30 V cone voltage applied in both modes. The source and desolvation temperatures were set at 120 °C and 400 °C, respectively, and the desolvation flow rate was 950 L/Hr.
For data analysis and identification, the retention time alignment, peak picking and database searching were accomplished using Progenesis QI (version 2.1, Nonlinear Dynamics, Newcastle, UK). Lipids were identified using (i) MetaScope by searching compound features against LIPID MAPS (http://www.lipidmaps.org/) and (ii) LipidBlast (http://fiehnlab.ucdavis.edu/). The search parameter used for precursor ion mass accuracy was set at 5 ppm and the theoretical fragmentation ion mass accuracy was set at 10 ppm. Reported compound identifications were scored and filtered by isotope similarity ≥90%. Identified compounds with abundance >100 and abundance variation among three replicates with <30% coefficient of variation were investigated.
Bioinformatics analysis
All genes involved in lipid metabolism were gathered using QuickGO, a web-based tool for gene ontology searching. The term GO:0006629 assigns lipid metabolic processes. Gene products belonging to T. papuae were collated. The obtained protein sequences were subjected to the Basic Local Alignment Search Tool (BLAST) and used to search the human non-redundant protein, Trichinella and C. elegans sequence databases. Any proteins with less than a 50% sequence similarity to human proteins were reported as being distinctive T. papuae proteins.
Acknowledgements
This study was supported by Goal-oriented Research Project Grant 2016 and ICTM grant of Mahidol University through Dr. Onrapak Reamtong.
Supplemenatry information
Author contributions
All authors participated in the design, interpretation and analysis of the study; S.M., P.T. P.S. and O.R. conducted and analysed the lipidomic experiments; P.A. maintained parasite life cycle; N.S. and O.R. performed bioinformatics analysis; All authors wrote, revised and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-67297-8.
References
- 1.Bruschi F, Murrell K. New aspects of human trichinellosis: the impact of new Trichinella species. Postgrad. Med. J. 2002;78:15–22. doi: 10.1136/pmj.78.915.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khumjui C, et al. Outbreak of Trichinellosis Caused by Trichinella papuae, Thailand, 2006. Emerg. Infect. Dis. 2008;14:1913–1915. doi: 10.3201/eid1412.080800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kusolsuk T, et al. The second outbreak of trichinellosis caused by Trichinella papuae in Thailand. Trans. R. Soc. Trop. Med. Hyg. 2010;104:433–437. doi: 10.1016/j.trstmh.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Feidas H, Kouam MK, Kantzoura V, Theodoropoulos G. Global geographic distribution of Trichinella species and genotypes. Infect. Genet. Evol. 2014;26:255–266. doi: 10.1016/j.meegid.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Gottstein B, Pozio E, Nöckler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin. Microbiol. Rev. 2009;22:127–145. doi: 10.1128/CMR.00026-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David J. & Petri W. Markell and Voge’s Medical Parasitology. Elsevier Saunders (2006).
- 7.Watt G, Saisorn S, Jongsakul K, Sakolvaree Y, Chaicumpa W. Blinded, placebo-controlled trial of antiparasitic drugs for trichinosis myositis. J. Infect. Dis. 2000;182:371–374. doi: 10.1086/315645. [DOI] [PubMed] [Google Scholar]
- 8.Pozio E, Sacchini D, Sacchi L, Tamburrini A, Alberici F. Failure of mebendazole in the treatment of humans with Trichinella spiralis infection at the stage of encapsulated larvae. Clin. Infect. Dis. 2001;32:638–642. doi: 10.1086/318707. [DOI] [PubMed] [Google Scholar]
- 9.Wat tG, Silachamroon U. Areas of uncertainty in the management of human trichinellosis: a clinical perspective. Expert. Rev. Anti Infect. Ther. 2004;2:649–652. doi: 10.1586/14787210.2.4.649. [DOI] [PubMed] [Google Scholar]
- 10.Saïdani N, Grando D, Valadié H, Bastien O, Maréchal E. Potential and limits of in silico target discovery–case study of the search for new antimalarial chemotherapeutic targets. Infect. Genet. Evol. 2009;9:359–367. doi: 10.1016/j.meegid.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Carrasco-Pancorbo A, Navas-Iglesias N, Cuadros-Rodríguez L. From lipids analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Trends Anal. Chem. 2009;28:393–403. [Google Scholar]
- 12.Zheng L, et al. Profiling of lipids in Leishmania donovani using hydrophilic interaction chromatography in combination with Fourier transform mass spectrometry. Rapid Commun. Mass. Spectrom. 2010;24:2074–2082. doi: 10.1002/rcm.4618. [DOI] [PubMed] [Google Scholar]
- 13.Besteiro S, Bertrand-Michel J, Lebrun M, Vial H, Dubremetz JF. Lipidomic analysis of Toxoplasma gondii tachyzoites rhoptries: further insights into the role of cholesterol. Biochem. J. 2008;415:87–96. doi: 10.1042/BJ20080795. [DOI] [PubMed] [Google Scholar]
- 14.Wang T, et al. The developmental lipidome of Haemonchus contortus. Int. J. Parasitol. 2018;48:887–895. doi: 10.1016/j.ijpara.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Botté C, et al. Subcellular localization and dynamics of a digalactolipid-like epitope in Toxoplasma gondii. J. Lipid Res. 2008;49:746–762. doi: 10.1194/jlr.M700476-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Debierre-Grockiego F, et al. Binding of Toxoplasma gondii glycosylphosphatidylinositols to galectin-3 is required for their recognition by macrophages. J. Biol. Chem. 2010;285:32744–32750. doi: 10.1074/jbc.M110.137588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boutlis CS, et al. Antibodies to Plasmodium falciparum glycosylphosphatidylinositols: inverse association with tolerance of parasitemia in Papua New Guinean children and adults. Infect. Immun. 2002;70:5052–5057. doi: 10.1128/IAI.70.9.5052-5057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caldarelli SA, et al. Exploration of potential prodrug approach of the bis-thiazolium salts T3 and T4 for orally delivered antimalarials. Bioorg Med. Chem. Lett. 2010;20:3953–3956. doi: 10.1016/j.bmcl.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Rakotomanga M, Saint-Pierre-Chazalet M, Loiseau PM. Alteration of fatty acid and sterol metabolism in miltefosine-resistant Leishmania donovani promastigotes and consequences for drug-membrane interactions. Antimicrob. Agents Chemother. 2005;49:2677–2686. doi: 10.1128/AAC.49.7.2677-2686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saint-Pierre-Chazalet M, et al. Membrane sterol depletion impairs miltefosine action in wild-type and miltefosine-resistant Leishmania donovani promastigotes. J. Antimicrob. Chemother. 2009;64:993–1001. doi: 10.1093/jac/dkp321. [DOI] [PubMed] [Google Scholar]
- 21.Hac-Wydro K, Dynarowicz-Łatka P, Zuk R. Langmuir monolayer study toward combined antileishmanian therapy involving amphotericin B and edelfosine. J. Phys. Chem. B. 2009;113:14239–14246. doi: 10.1021/jp9032996. [DOI] [PubMed] [Google Scholar]
- 22.López-Martín C, Pérez-Victoria JM, Carvalho L, Castanys S, Gamarro F. Sitamaquine sensitivity in Leishmania species is not mediated by drug accumulation in acidocalcisomes. Antimicrob. Agents Chemother. 2008;52:4030–4036. doi: 10.1128/AAC.00964-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K. HMDB 4.0 — The Human Metabolome Database for 2018. Nucleic Acids Res. 2018;46:D608–617. doi: 10.1093/nar/gkx1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han X, Gross RW. Electrospray ionization mass spectroscopic analysis of human erythrocyte plasma membrane phospholipids. Proc. Natl Acad. Sci. USA. 1994;91:10635–10639. doi: 10.1073/pnas.91.22.10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han X, Gross RW. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass. Spectrom. Rev. 2005;24:367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- 26.Hermansson M, Hokynar K, Somerharju P. Mechanisms of glycerophospholipid homeostasis in mammalian cells. Prog. Lipid Res. 2011;50:240–257. doi: 10.1016/j.plipres.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Patnaik PK, et al. Molecular species analysis of phospholipids from Trypanosoma brucei bloodstream and procyclic forms. Mol. Biochem. Parasitol. 1993;58:97–105. doi: 10.1016/0166-6851(93)90094-e. [DOI] [PubMed] [Google Scholar]
- 28.Giera M, et al. The Schistosoma mansoni lipidome: Leads for immunomodulation. Anal. Chim. Acta. 2018;11:107–118. doi: 10.1016/j.aca.2017.11.058. [DOI] [PubMed] [Google Scholar]
- 29.Wewer V, et al. Lipid profiling of the filarial nematodes Onchocerca volvulus, Onchocerca ochengi and Litomosoides sigmodontis reveals the accumulation of nematode-specific ether phospholipids in the host. Int. J. Parasitol. 2017;47:903–912. doi: 10.1016/j.ijpara.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young BW, Podesta RB. Major phospholipids and phosphatidylcholine synthesis in adult Schistosoma mansoni. Mol. Biochem. Parasitol. 1982;5:165–172. doi: 10.1016/0166-6851(82)90018-4. [DOI] [PubMed] [Google Scholar]
- 31.Gobert GN, Stenzel DJ, McManus DP, Jones MK. The ultrastructural architecture of the adult Schistosoma japonicum tegument. Int. J. Parasitol. 2003;33:1561–1575. doi: 10.1016/s0020-7519(03)00255-8. [DOI] [PubMed] [Google Scholar]
- 32.Wharton DA, Petrone L, Duncan A, McQuillan AJ. A surface lipid may control the permeability slump associated with entry into anhydrobiosis in the plant parasitic nematode Ditylenchus dipsaci. J. Exp. Biol. 2008;211:2901–2908. doi: 10.1242/jeb.020743. [DOI] [PubMed] [Google Scholar]
- 33.Goodridge HS, et al. Phosphorylcholine mimics the effects of ES-62 on macrophages and dendritic cells. Parasite Immunol. 2007;29:127–137. doi: 10.1111/j.1365-3024.2006.00926.x. [DOI] [PubMed] [Google Scholar]
- 34.Ball DH, Al-Riyami L, Harnett W, Harnett MM. IL-33/ST2 signalling and crosstalk with FcεRI and TLR4 is targeted by the parasitic worm product, ES-62. Sci. Rep. 2018;8:4497. doi: 10.1038/s41598-018-22716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Riyami L, et al. Designing anti-inflammatory drugs from parasitic worms: a synthetic small molecule analogue of the Acanthocheilonema viteae product ES-62 prevents development of collagen-induced arthritis. J. Med. Chem. 2013;56:9982–10002. doi: 10.1021/jm401251p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berry KA, Murphy RC. Analysis of cell membrane aminophospholipids as isotope-tagged derivatives. J. Lipid Res. 2005;46:1038–1046. doi: 10.1194/jlr.M500014-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Colombo S, et al. Modulation of the inflammatory response of immune cells in human peripheral blood by oxidized arachidonoyl aminophospholipids. Arch. Biochem. Biophys. 2018;15:64–71. doi: 10.1016/j.abb.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Smith TK, et al. Parasite and mammalian GPI biosynthetic pathways can be distinguished using synthetic substrate analogues. EMBO J. 1997;16:6667–6675. doi: 10.1093/emboj/16.22.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferguson MA, et al. Glycosyl-phosphatidylinositol molecules of the parasite and the host. Parasitology. 1994;108:S45–54. doi: 10.1017/s0031182000075715. [DOI] [PubMed] [Google Scholar]
- 40.Naik RS, Krishnegowda G, Ockenhouse CF, Gowda DC. Naturally elicited antibodies to glycosylphosphatidylinositols (GPIs) of Plasmodium falciparum require intact GPI structures for binding and are directed primarily against the conserved glycan moiety. Infect. Immun. 2006;74:1412–1415. doi: 10.1128/IAI.74.2.1412-1415.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferguson MA. Glycosylphosphatidylinositol biosynthesis validated as a drug target for African sleeping sickness. Proc. Natl Acad. Sci. USA. 2000;97:10673–10675. doi: 10.1073/pnas.97.20.10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinoshita T, Inoue N. Dissecting and manipulating the pathway for glycosylphosphatidylinositol-anchor biosynthesis. Curr. Opin. Chem. Biol. 2000;4:632–638. doi: 10.1016/s1367-5931(00)00151-4. [DOI] [PubMed] [Google Scholar]
- 43.de Macedo CS, Shams-Eldin H, Smith TK, Schwarz RT, Azzouz N. Inhibitors of glycosyl-phosphatidylinositol anchor biosynthesis. Biochimie. 2003;85:465–472. doi: 10.1016/s0300-9084(03)00065-8. [DOI] [PubMed] [Google Scholar]
- 44.Quittnat F, et al. On the biogenesis of lipid bodies in ancient eukaryotes: synthesis of triacylglycerols by a Toxoplasma DGAT1‐related enzyme. Mol. Biochem. Parasitol. 2004;138:107–122. doi: 10.1016/j.molbiopara.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Bisanz C, et al. Toxoplasma gondii acyl-lipid metabolism: de novo synthesis from apicoplast-generated fatty acids versus scavenging of host cell precursors. Biochem. J. 2006;394:197–205. doi: 10.1042/BJ20050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang K, Bangs JD, Beverley SM. Sphingolipids in parasitic protozoa. Adv. Exp. Med. Biol. 2010;688:238–248. doi: 10.1007/978-1-4419-6741-1_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nomura KH, et al. Ceramide glucosyltransferase of the nematode Caenorhabditis elegans is involved in oocyte formation and in early embryonic cell division. Glycobiology. 2011;21:834–848. doi: 10.1093/glycob/cwr019. [DOI] [PubMed] [Google Scholar]
- 48.Sonda S, Stefanic S, Hehl AB. A sphingolipid inhibitor induces a cytokinesis arrest and blocks stage differentiation in Giardia lamblia. Antimicrob. Agents Chemother. 2008;52:563–569. doi: 10.1128/AAC.01105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Kleij D, et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 2002;277:48122–48129. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 50.Baumruker T, Billich A, Brinkmann V. FTY720, an immunomodulatory sphingolipid mimetic: translation of a novel mechanism into clinical benefit in multiple sclerosis. Expert. Opin. Investig. Drugs. 2007;16:283–289. doi: 10.1517/13543784.16.3.283. [DOI] [PubMed] [Google Scholar]
- 51.Barrett-Bee K. & Ryder, N. Biochemical aspects of ergosterol biosynthesis inhibition. J Sutcliffe and N H Georgopapadakou 410–436 (1992).
- 52.Urbina JA, et al. Mevinolin (lovastatin) potentiates the antiproliferative effects of ketoconazole and terbinafine against Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Antimicrob. Agents Chemother. 1993;37:580–591. doi: 10.1128/aac.37.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mondal M, Kundu JK, Misra KK. Variation in lipid and fatty acid uptake among nematode and cestode parasites and their host. domestic fowl: host–parasite interaction. 2016;40:1494–1518. doi: 10.1007/s12639-015-0718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garofalo A, et al. The FAR protein family of the nematode. J. Biol. Chem. 2003;278:8065–8074. doi: 10.1074/jbc.M206278200. [DOI] [PubMed] [Google Scholar]
- 55.Macieira S, Zhang J, Velarde M, Buckel W, Messerschmidt A. Crystal structure of 4-hydroxybutyrate CoA-transferase from Clostridium aminobutyricum. Biol. Chem. 2009;390:1251–1263. doi: 10.1515/BC.2009.147. [DOI] [PubMed] [Google Scholar]
- 56.Larry, R. S. & Janovay, J. Foundations of Parasitology (7th ed.). McGraw-Hill 405–407 (2005).
- 57.Suresh CG, et al. Penicillin V acylase crystal structure reveals new Ntn-hydrolase family members. Nat. Struct. Mol. Biol. 1999;6:414–416. doi: 10.1038/8213. [DOI] [PubMed] [Google Scholar]
- 58.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signaling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 59.Ghannoum MA. Extracellular phospholipases as universal virulence factor in pathogenic fungi. Nihon Ishinkin Gakkai Zasshi. 1998;39:55–59. doi: 10.3314/jjmm.39.55. [DOI] [PubMed] [Google Scholar]
- 60.Hänel H, Kirsch R, Schmidts HL, Kottmann H. New systematically active antimycotics from the beta-blocker category. Mycoses. 1995;38:251–264. doi: 10.1111/j.1439-0507.1995.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 61.Hu F, et al. Molecular characterization of a novel Clonorchis sinensis secretory phospholipase A(2) and investigation of its potential contribution to hepatic fibrosis. Mol. Biochem. Parasitol. 2009;167:127–134. doi: 10.1016/j.molbiopara.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 62.Singh P, et al. Role of a patatin-like phospholipase in Plasmodium falciparum gametogenesis and malaria transmission. Proc. Natl Acad. Sci. USA. 2019;116:17498–17508. doi: 10.1073/pnas.1900266116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernandes, A. C. S. et al. Endocytosis and Exocytosis in Leishmania amazonensis Are Modulated by Bromoenol Lactone. Front Cell Infect Microbiol. 10–39 (2020). [DOI] [PMC free article] [PubMed]
- 64.Burda, P. C. et al. Plasmodium phospholipase is involved in disruption of the liver stage parasitophorous vacuole membrane. PLoS Pathog. 11 (2015) [DOI] [PMC free article] [PubMed]
- 65.Kong AM, et al. Cloning and characterization of a 72-kDa inositol-polyphosphate 5-phosphatase localized to the Golgi network. J. Biol. Chem. 2000;275:24052–24064. doi: 10.1074/jbc.M000874200. [DOI] [PubMed] [Google Scholar]
- 66.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 67.Tipthara P, et al. C2., Boehm, B. O. Global Profiling of Metabolite and Lipid Soluble Microbial Products in Anaerobic Wastewater Reactor Supernatant Using UPLC-MSE. J. Proteome Res. 2017;16:559–570. doi: 10.1021/acs.jproteome.6b00681. [DOI] [PubMed] [Google Scholar]