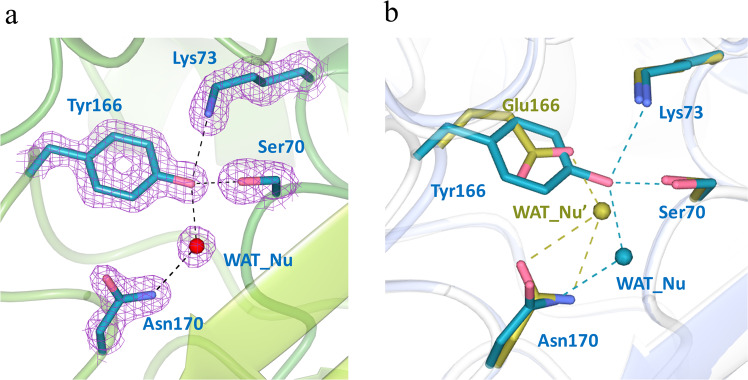
Figure 3.
The apo structure of Glu166Tyr reveals an extensive hydrogen bonding network for Tyr166 and a centrally located water molecule in the active site. (a) Tyr166 forms a network of hydrogen bonds with other key catalytic residues and a hydrolytic water molecule (WAT_Nu) in the active site. Fo-Fc omit map (purple) is drawn in mesh format and contoured at 2.0 σ. The dashed lines indicate hydrogen bonds. (b) Superposition of Glu166Tyr active site (cyan) with that of the wild-type (golden). WAT_Nu: the putative hydrolytic water molecule in the wild-type structure. Figures are made using CCP4mg.