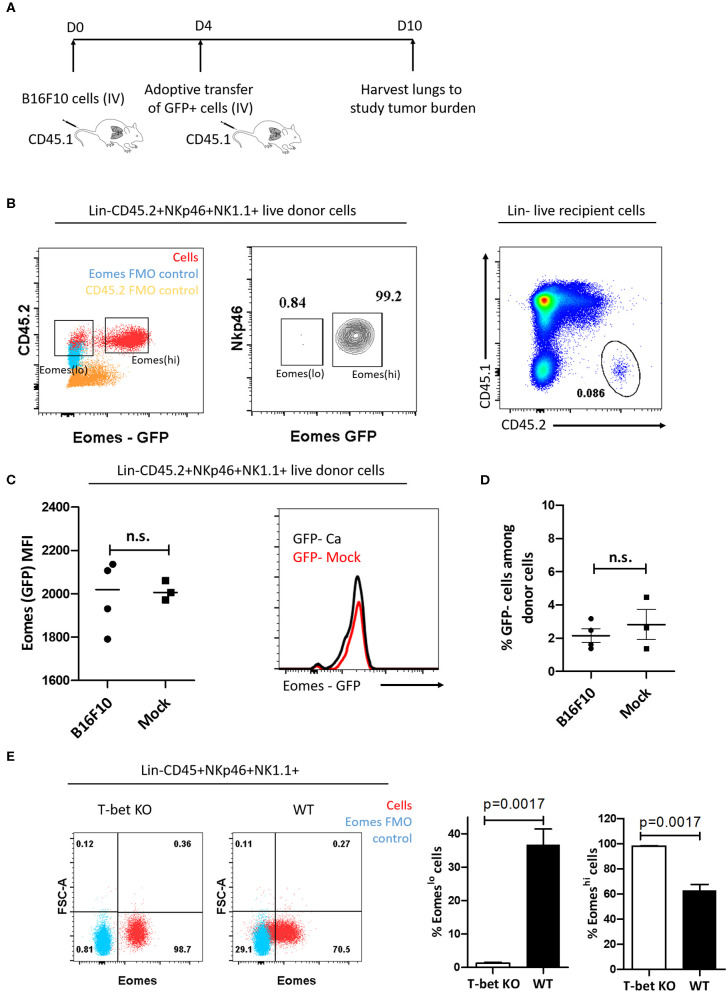
Figure 3.
Eomeslo and Eomeshi subsets correspond to different cell lineages. (A) Scheme of adoptive transfer of Eomeshi cells into CD45.1 mice bearing cancer (relative to mock). After injection of B16F10 cells (day 0), Eomeshi cells were harvested from donor Eomes-GFP mice and adoptively transferred into recipient mice at day 4. The mice were then sacrificed at day 10 and lungs were harvested and analyzed. (B) Eomeslo and Eomeshi cells in Eomes-GFP mice (Red) overlaid with Eomes FMO (blue) (left panel). Two distinct populations can be seen upon running live cells under flow cytometer; Sorting efficiency ~99% middle panel); Donor cells after transfer of CD45.2+ Eomeshi-GFP+ cells into CD45.1 mice (right panel). (C) Eomes (MFI) after transfer of donor derived Eomeshi GFP+ cells in cancer-bearing (B16F10) and cancer-lacking (mock) hosts at day 10. No significant difference in Eomes MFI was observed between cancer and mock mice. (D) Frequency of Eomeslo GFP− cells amongst donor NK cells at day 10 post-injection of B16F10 cells (E) Flow plots and graphs show near absence of Eomeslo cells in T-bet KO mice (~0.8%) compared to WT mice (~29.1%), indicating that T-bet is needed for development of Eomeslo cell development (Red—NKp46+NK1.1+ cells; Blue—Eomes FMO). MFI is Mean Fluorescence Intensity, n = 3–4 for each group. Results are representative of three independent repeats; data are presented as mean ± s.e.m.; Significance was tested using two-tailed students' t-test.