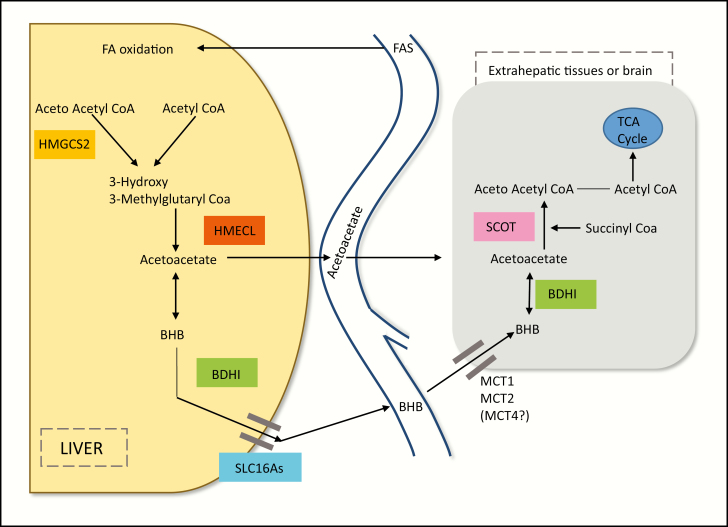
Figure 1.
The biochemistry of ketogenesis. Prolonged glucose restriction leads to an increased glucagon insulin ratio, relieving the inhibition of adipose triglyceride lipase and hormone-sensitive lipase, which are key enzymes in the production of free fatty acids (FFAs) in adipocytes and their subsequent release into the peripheral circulation. Decreased levels of insulin and glucose also combine to relieve the inhibition of carnitine acyltransferase 1 in the liver, which governs the uptake of FFAs into mitochondria, and to increase levels of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) while reducing levels of oxaloacetate, as it is used as a precursor for the manufacture of glucose. The net effect of these changes is increased glucagon-mediated transport of FFAs into the liver and increased uptake into mitochondria where they are used for the manufacture of acetyl coenzyme A (acetyle-CoA). In normal conditions, this would enter the tricarboxylic acid (TCA) cycle, but in an environment of reduced oxaloacetate the only metabolic pathway open to the molecule is ketogenesis involving the formation of ketone bodies via a series of steps. In the first of these reactions, Acetyl-CoA (AcCoA) is converted to acetoacetyl CoA in a reaction enabled by 3-Ketothiolase. This molecular intermediate is then converted to HMG CoA by HMG-CoA synthase, which is constitutively expressed in mitochondria. The final reaction in this pathway is the cleavage of HMG-CoA by HMG-CoA Lyase to produce acetoacetate. beta-hydroxybutyrate (BHB) may then be formed by the reversible reduction of acetoacetate mediated by 3-hydroxybutyrate dehydrogenase, and acetone may be produced by the thermodynamically favorable decarboxylation of aceoaetic acid (AA). Egress of these ketone bodies from the liver is facilitated by the transporter Solute Carrier Family 16, Member 6 (SLC16A6). Their subsequent entry into peripheral tissues and brain facilitated by monocarboxylic acid transporters ultimately serves as a source of AcCoA for the TCA cycle. Once in situ, BHB may be reconverted to acetoacetate in a reaction enabled by the same enzyme. However, from that point, ketolysis and utilization of ketone bodies display major biochemical differences compared with ketogenesis. In particular, Succinyl-CoA transfers its CoA group to acetoacetate to produce acetoacetyl-CoA in a reaction enabled by the enzyme succinyl-CoA:3-ketoacid coenzyme A transferase (also known as OXCT1 or SCOT), bypassing the irreversible step in ketogenesis catalyzed by HMG-CoA synthase and thereby preventing the development of a futile cycle of hepatic BHB synthesis and utilization.