Abstract
Mitochondria are important regulators of cerebral vascular function in health and disease, but progress in understanding their roles has been hindered by methodological limitations. We report the first in vivo imaging of mitochondria specific to the cerebral endothelium in real time in the same mouse for extended periods. Mice expressing Dendra2 fluorescent protein in mitochondria (mito-Dendra2) in the cerebral vascular endothelium were generated by breeding PhAM-floxed and Tie2-Cre mice. We used mito-Dendra2 expression, cranial window implantation, and two-photon microscopy to visualize mitochondria in the cerebral vascular endothelium of mice. Immunohistochemistry and mitochondrial staining were used to confirm the localization of the mitochondrial signal to endothelial cells and the specificity of mito-Dendra2 to mitochondria. Mito-Dendra2 and Rhodamine B-conjugated dextran allowed simultaneous determinations of mitochondrial density, vessel diameters, area, and mitochondria-to-vessel ratio in vivo, repeatedly, in the same mouse. Endothelial expression of mito-Dendra2 was confirmed in vitro on brain slices and aorta. In addition, we observed an overlapping mito-Dendra2 and Chromeo mitochondrial staining of cultured brain microvascular endothelial cells. Repeated imaging of the same location in the cerebral microcirculation in the same mouse demonstrated stability of mito-Dendra2. While the overall mitochondrial signal was stable over time, mitochondria within the same endothelial cell were mobile. In conclusion, our results indicate that the mito-Dendra2 signal and vascular parameters are suitable for real-time and longitudinal examination of mitochondria in vivo in the cerebral vasculature of mice.
NEW & NOTEWORTHY We introduce an innovative in vivo approach to study mitochondria in the cerebral circulation in their physiological environment by demonstrating the feasibility of long-term imaging and three-dimensional reconstruction. We postulate that the appropriate combination of Cre/Lox system and two-photon microscopy will contribute to a better understanding of the role of mitochondria in not only endothelium but also the different cell types of the cerebral circulation.
Keywords: cerebral circulation, endothelium, in vivo, mitochondria, two-photon microscopy
INTRODUCTION
Mitochondria play critical roles in the physiology and pathophysiology of endothelial cells in an organ-dependent manner (3). Mitochondria in the cerebral vascular endothelium produce ATP for normal cellular functioning, including maintenance of the physical and transport properties of the blood-brain barrier as well as providing energy for vascular remodeling, repair, and angiogenesis in response to changing physiological conditions and disease (9, 11, 20, 22). Mitochondria also produce reactive oxygen species, which, depending upon levels and cellular status, have normal regulatory or detrimental effects on endothelial cell function. Less well known are the actions of small, biologically active peptides encoded and synthesized by mitochondria that promote resistance to stress (20).
Rather than static entities, mitochondria are dynamic organelles, which adjust to changing physiological and pathological conditions (2, 12, 40). Previous studies of mitochondria in the cerebral vascular endothelium were performed in cultured cells or using ex vivo approaches (10, 26). Thus, little is known concerning normal mitochondrial behavior and mechanisms of mitochondrial regulation under different conditions in the cerebral vascular endothelium in living animals. This lack of in vivo study of mitochondrial characteristics in the cerebral vascular endothelium has restricted our understanding of the roles played by mitochondria in health and during disease states and has hindered the development of novel therapeutics. To remedy this deficiency, we bred PhAM-floxed (31) and Tie2-Cre (21) mice to generate offspring that expressed genetically labeled mitochondria in the endothelium via Dendra2, a genetically encoded fluorescent protein (17, 24) (mito-Dendra2), and imaged the mitochondria through a cranial window using two-photon microscopy. We have demonstrated that visualizing mitochondria in the vascular endothelium using in vivo two-photon microscopy allows for real-time and longitudinal examination of mitochondria in the cerebral vasculature of living mice.
MATERIALS AND METHODS
Animals
Mice expressing mito-Dendra2 (n = 5 females and 2 males) were generated by breeding PhAM-floxed female and Tie2-Cre male mice, obtained from Jackson Laboratory: B6;129S-Gt(ROSA)26Sortm1(CAG-COX8A/Dendra-2)Dcc/J, Stock No: 018385, n = 3; B6.Cg-Tg(Tek-cre)1Ywa/J; Stock No: 008863, n = 3; respectively (21, 31). Mito-Dendra2 mice were first developed by Dr. Chan’s laboratory by inserting an expression cassette containing Dendra2 targeted to the mitochondrial matrix into the Rosa26 locus (31). Tie2-Cre mice, with endothelial-specific promoter/enhancer-driven expression of the Cre recombinase activity, were developed by Dr. Yanagisawa’s laboratory (21). Heterozygous males were used to transmit the Tie2-Cre allele (Cre+/−), whereas females were homozygous for mito-Dendra2 (Flox+/+). Only first-generation offspring were used in our studies. Approximately half of the offspring exhibited the desired Flox+Cre+ genotype, resulting in a green mitochondrial signal in the endothelium. Genotyping was done on DNA extracted from tail samples after overnight digestion at 55°C using proteinase K (No. P2308; Sigma-Aldrich, St. Louis, MO) and direct PCR (No. 101-T; Viagen Biotech; Los Angeles, CA) mixtures. Proteinase K was then deactivated by increasing the temperature from 55 to 85°C for 1 hr. DNA content was determined using a NanoDrop 2000/Spectrophotometer. The following are the primer combinations sequences: 5′→ 3′, 1) GCGGTCTGGCAGTAAAAACTATC, GTGAAACAGCATTGCTGTCACTT, CTAGGC CACAGAATTGAAAGATCT, and GTAGGTGGAAATTCTAGCATCATCC; 2) TCAATGGGCGGGGGTCGTT, TCCTGGCTTCTGAGGACCGC, and TTCCCCTGCAGGACAACGCC; and 3) CAACGTGCTGGTTATTGTGC, CTCCAAGAAACGAAAAGGCC, and CCAACGAATGGATCTTGGCG (Fisher Scientific, Waltham, MA; primer 1 for Cre, primers 2 and 3 for PhaM floxed), Platinum Hot Start PCR Master Mix (2×), containing nuclease-free water, Platinum PCR 2× Master Mix, Platinum GC ProFlex PCR System Enhancer, and 25 μM of MgCl2 were used for the PCR reactions (No. 13000013; Invitrogen/Fisher Scientific, Waltham, MA). PCR conditions (temperature, time, cycle) are as follows: stage 1, 94°C, 2 m, 1×; stage 2, 94°C, 0.5 m, 35×; 58°C, 0.5 m, 35×; and 72°C, 0.5 m, 35×; stage 3, 72°C, 10 m, 1×, and 4°C. The amplified samples were then separated on agarose gel and visualized with ImageQuant LAS 500.
Animals were housed in the vivarium of the Department of Comparative Medicine at Tulane University with access to food and water ad libitum. Animal procedures and protocols were approved by the Institutional Animal Care and Use Committee of Tulane University and performed in accordance with the “Animal Research: Reporting of In Vivo Experiments” and National Institutes of Health guidelines.
Immunohistochemistry
Animals were deeply anesthetized and euthanized using isoflurane and a rodent guillotine (for decapitation). The brain and aorta were removed after euthanasia and processed for immunohistochemistry to confirm the endothelial localization of mito-Dendra2 signal (Fig. 1). Tissue samples were maintained in 4% paraformaldehyde-PBS (Sc-281692; ChemCruz, Dallas, TX) for 24 h at 4°C and then transferred into a 30% sucrose-PBS solution (S3-212; Fisher Scientific, Waltham, MA). Afterward, the OCT (No. 4583; Tissue-Tek) embedded tissue was cut using a cryostat. The air-dried slides were incubated in Diva Decloaker RTU (DV2004MM; Biocare Medical, Pacheco, CA) for 10 min at 37°C, followed by a 2-min incubation in 3% H2O2 at room temperature. PBS and dH2O were used to wash the samples between steps. The tissue was next incubated in 0.05% Tween-20-PBS at room temperature for 5 min and blocked for 10 min at room temperature using Background Sniper (BS966L; Biocare Medical, Pacheco, CA). While protected from light, the tissue slices were incubated with primary antibody overnight at 4°C and then at room temperature for 1 h with the respective secondary antibodies. Van Gogh Diluent (PD902L; Biocare Medical, Pacheco, CA) was used to dilute Rabbit Anti-CD31 (ab28364; Abcam, Cambridge, MA) antibody at 1:50 to label the endothelium. Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 555 (A-21428; Invitrogen, Waltham, MA) secondary antibody, was diluted in fluorescent antibody diluent (FAD901L; Biocare Medical, Pacheco, CA) at 1:200. DAPI (H-1200-10; Vector Laboratories, Burlingame, CA) was used to stain the nuclei. Olympus BX51 microscope with a DP80 color camera, UPlanFl 40×/0.75, ∞/0.17 and UPlanFl 60×/1.25 Oil Iris Japan objectives, and cellSens Dimension software were used to obtain the images.
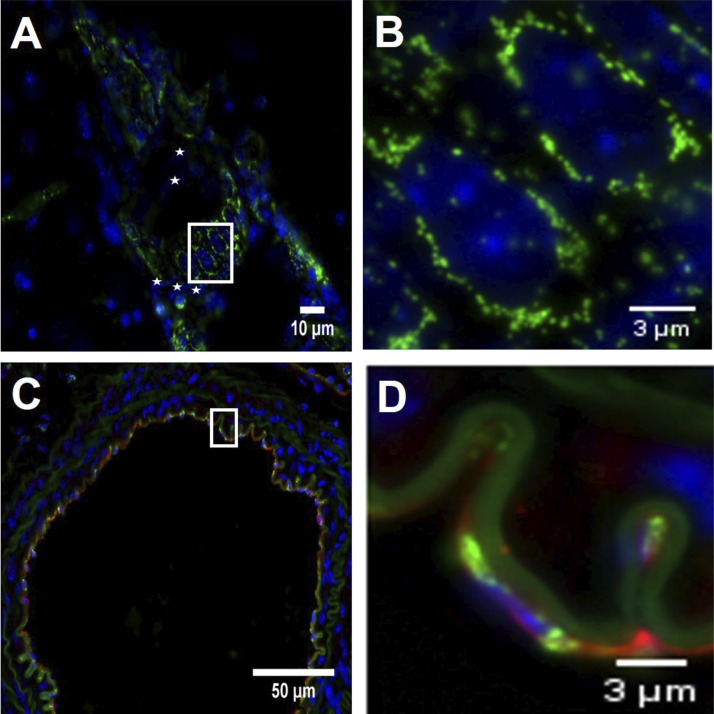
Fig. 1.
Endothelial mito-Dendra2 signal. A: representative image shows mito-Dendra2 signal (green) around DAPI-stained endothelial cell nuclei (blue) in a large brain vessel at 60× magnification, positioned parallel to the flow direction, whereas the smooth muscle cell nuclei, marked by white asterisks, are perpendicular to them. Scale bar indicates 10 μm. B: enlarged white-boxed section from A with a scale bar of 3 μm. C: image of an aortic section, taken at 40× magnification, shows the colocalization of CD31 endothelial marker (red) and mito-Dendra2 (green) mitochondrial signal. Blue indicates DAPI-stained cell nuclei. Scale bar indicates 50 μm. D: enlarged white-boxed section from C with a scale bar of 3 μm. This enlarged section of the aorta shows that the green mitochondrial mito-Dendra2 signal is localized around a CD31 positive endothelial cell (red) nucleus (blue).
Mouse Brain Microvascular Endothelial Cells Isolation and Mitochondrial Staining
Mouse brain microvascular endothelial cells (MBMECs) were cultured from isolated microvessels after mice euthanasia and decapitation based on the protocol described by Ruck, et al. (33), and Chromeo Live Cell Mitochondrial Staining Kit (No. 15005; Active Motif, Carlsbad, CA) was used to confirm the mitochondrial-specific location of the mito-Dendra2 signal on cultured MBMECs. Briefly, collagenase and DNaseI from bovine pancreas (0.4 ng/ml, No. LS004177, Worthington Biochemical, Lakewood, NJ; 0.01 mg/ml, No. 1128492001, Roche, Sigma-Aldrich, St. Louis, MO; respectively) were used to digest the minced cortexes. The homogenate was then centrifuged and resuspended in 20% BSA-DMEM (A3059; Sigma-Aldrich, St. Louis, MO; 11995-065; Gibco, Waltham, MA; respectively) to remove the myelin. Microvessels containing the microvascular endothelial cells were separated via Percoll gradient centrifugation and then plated on fibronectin-collagen IV-coated dishes in media with puromycin (10 ng/ml; ant-pr-1; Invitrogen, Waltham, MA), which was changed to antibiotic free media after 48 h. Images were captured using an EVOS FL Cell Imaging System with an EVOS AMEP 4699 40× objective (Fig. 2).
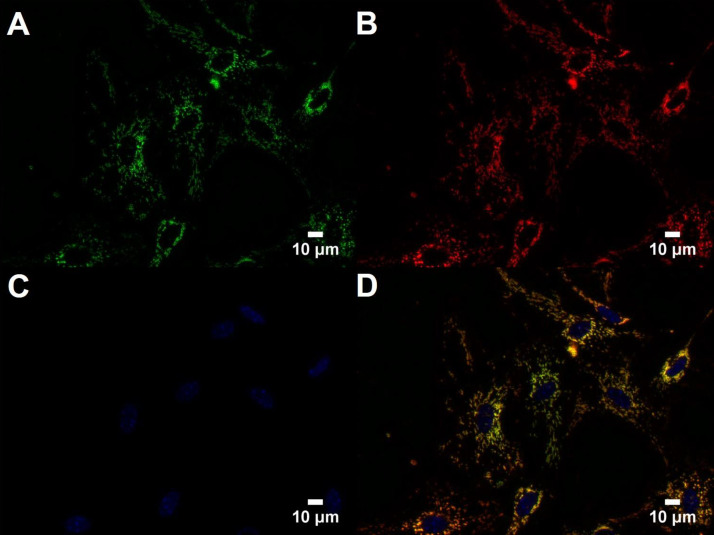
Fig. 2.
Mito-Dendra2 signal in cultured brain microvascular endothelial cells. A–C: images of mito-Dendra2 signal (green; A), Chromeo-labeled mitochondria (red; B), and cell nuclei (C) stained with Hoechst (blue) were taken at 40× magnification. D: merged image shows overlapping mito-Dendra2 and Chromeo signals in cultured brain microvascular endothelial cells. Scale bars indicate 10 μm.
Surgical Procedures
A cranial window was placed on the top of the skull, as described by Mostany and Portera-Cailliau (18, 29). Briefly, anesthesia was induced by an isoflurane (No. 502017; VetOne, Boise, ID) - oxygen mixture at 5% and maintained at 1.5%, respectively. Sterility was maintained during surgical procedures. A heating pad and thermometer were used to control the animal’s core temperature at 37°C. Eye ointment was used to lubricate the eyes. Dexamethasone (0.2 mg/kg sc; No. 501012; VetOne, Boise, ID) and carprofen (5 mg/kg sc; No. 141-199; Zoetis, Parsippany, NJ) were injected before the surgical procedure to prevent brain edema and inflammation. A piece of shaved, disinfected skin was removed from the skull with lidocaine (2%; No. 510212; VetOne, Boise, ID) used for topical analgesia. The skull over the middle cerebral artery area was removed (~4 mm in diameter) using a dental drill for the repeated two-photon measurements. Afterward, a 5 mm coverslip was secured to the head of the mouse using cyanoacrylate-based glue and dental acrylic (No. 64-0700; Warner Instruments, Hamden, CT), and a titanium bar was then secured on the head using the same adhesive.
Two-Photon Imaging of the Cerebral Vasculature and Endothelial Mitochondria
High-resolution in vivo two-photon imaging was performed with a SliceScope microscope (Scientifica, Clarksburg, NJ), equipped with a Chameleon Ultra II Ti:sapphire laser (Coherent Inc., Santa Clara, CA), tuned to 875 nm, with 525/50- and 620/60-nm emission filters for the green and red channels, respectively, and a 565LP dichroic mirror. Images were taken using an Olympus 40 × 0.80 numerical aperture water immersion objective. First imaging session was performed 2 wk after cranial window surgery. Animals were secured to the microscope stage via a titanium bar and imaged under light isoflurane anesthesia. They were kept on a heating pad, and body temperature was maintained at 37°C. Rhodamine B isothiocyanate-70 kDa dextran (50 μl of 5% wt/vol; No. R9379; Sigma-Aldrich, St. Louis, MO) was used to label the blood plasma via retro-orbital injection. Mice were imaged in a random order throughout the imaging sessions.
Images for mitochondrial and vessel area determination.
Animals were imaged four times starting at zero time point (0 wk) and then 2, 4, and 12 wk (Fig. 3). With a start at 40–50 μm below the brain surface, stacks of 100 consecutive images were taken at 1-μm increments (Z step). Images were acquired using 40× magnification in combination with a 1× digital zoom or at 40× magnification combined with a 3× digital zoom. Image dimensions taken at 40× magnification with a 1× digital zoom (1× images) were 512 × 512 pixels, 368 × 377 μm, and 0.72 μm/pixel. Image dimensions taken at 40× magnification with a 3× digital zoom (3× images) were 512 × 512 pixels, 125.5 × 126 μm, and 0.25 μm/pixel.
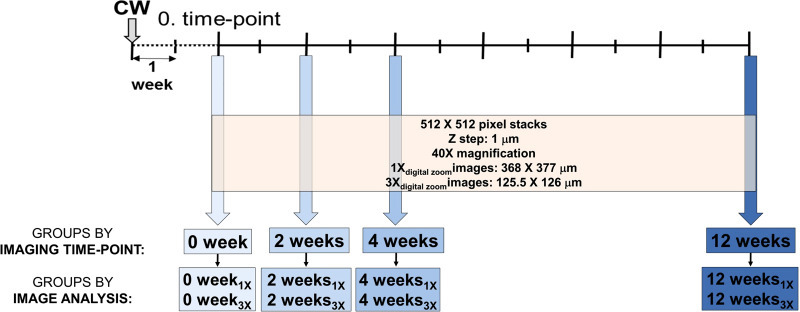
Fig. 3.
Schematic of 2-photon experimental design. Cranial window (CW) was implanted 2 wk before imaging. Imaging was performed at a 0 time point and 2, 4, and 12 wk from the 0 time point. Image groups are labeled by sequential-imaging time points: 0 time-point images represent group 0 wk, images taken 2 wk from 0 time point represent group 2 wk, images at 4 wk from 0 represent group 4 wk, and images taken 12 wk from 0 represent group 12 wk. During each imaging time point, images were taken at 40× magnification in combination with 1× or 3× digital zoom, resulting in the following subgroups: 0 wk1× and 0 wk3× within group 0 wk, 2 wk1× and 2 wk3× within group 2 wk, 4 wk1× and 4 wk3× within group 4 wk, and 12 wk1× and 12 wk3× within group 12 wk.
Images for mitochondrial quality control determination.
Time-lapse imaging was used to record the same vessel area at zero time point and within 30–60 min during one imaging session using 1× magnification (512 × 512 pixels, 368 × 377 μm, and 0.72 μm/pixel) and 3× magnification (512 × 512 pixels, 125.5 × 126 μm, and 0.25 μm/pixel). The mitochondrial signal was then compared in the two image stacks, frames by frame, to identify changes in mitochondria indicated by the change of green signal.
Image Analysis
Area determination.
Mitochondrial area, vessel area, and the mean vessel diameter were determined using ImageJ Fiji 1.52d software (34, 35). Mice were imaged four times, starting at 5 mo of age, represented by the zero time point (0 wk) and then 2, 4, and 12 wk, using 40× magnification with 1× digital zoom (0 wk1×, 2 wk1×, 4 wk1×, and 12 wk1×) or 3× digital zoom (0 wk3×, 2 wk3×, 4 wk3×, and 12 wk3×) (Fig. 3). Imaging sessions were conducted by two people who were not involved in data analysis. One mouse was excluded from data analysis since decreased cranial window quality prevented imaging after the second imaging session. Mitochondrial and vessel areas were determined using ImageJ 1.52d Particle Analyzer on binarized and maximum intensity-projected images (34, 35). For this analysis, three substacks were made of 1× image stacks to compensate for the decreased intensity associated with depth. Image stacks were opened in ImageJ with pixels as units (default opening, not scaled images). For scaling, we used the Analyze menu within ImageJ by clicking “set scale,” provided the distance in pixels (512), the known distance (at X1× image, 368; and Y1× image, 377; X3× image, 125.5; and Y3× image, 126), pixel aspect ratio (for 1× images, 0.976 and 0.996 for 3× images), as well as the unit (μm). The channels were then separated using the deinterleaving feature of ImageJ (image→stacks→tools→deinterleave→two channels; image No. 1 or green channel contained the mitochondrial signal, whereas image No. 2 or red channel represented the labeled blood plasma). The 3× images were deinterleaved, whereas 1× images were used to make substacks before deinterleaving (image→stacks→tools→make substacks; e.g.; images with 200 frames were divided as frames no. 1–66, 67–133, and 134–200). The brightness and contrast were then adjusted only for the mitochondrial images (image→adjust→brightness/contrast; minimum value was moved from 0 to 250 based on our observation that this change could be applied across the images and resulted in a decreased blurriness around the mitochondria). Afterward, these processed images were saved and binarized (process→binary→make binary; in a couple of cases, noisy images were depeckled via process→noise→despeckle) and then maximum projected (image→stacks, z projection→maximum intensity). Analysis was performed using the Particle analyzer (analyze→particle analysis with size of 0–784 pixel or 0–50 μm2 for mitochondrial images and 10/20, infinity pixel or 1.25, and infinity μm2 for vessel images). For the mean vessel diameter measurements, deinterleaved images (image→stacks→tools→deinterleave→save image No. 2 or the vessel image) were scaled according to the listed X and Y distances. Contrast and brightness were then adjusted using the auto function (image→adjust→brightness/contrast→auto→apply). The Tubeanalyst file was downloaded into the Fiji.app Plugins folder and opened via plugins→macros→Tubeanalyst (using the following settings: tube radius, 2; vessel radius, 2; vessel threshold, 8; minimal vessel volume, 90; dilate skeleton for viewing by, 1; and number of threads, 8) and used to determine the mean vessel diameter (in μm) (30). Vesselucida360 (MBF Bioscience, Williston, VT) was used for three-dimensional vessel and mitochondrial reconstruction (Fig. 4).
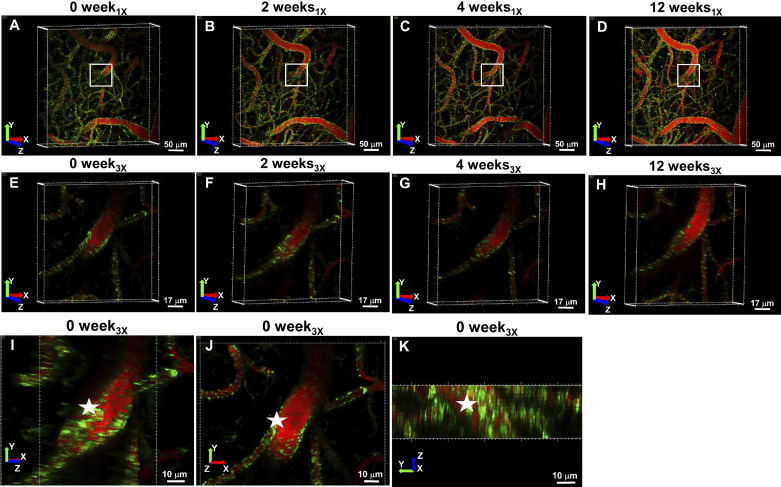
Fig. 4.
In vivo visualization of mitochondria and the cerebral vasculature over time. Green color indicates mito-Dendra2 signal, whereas red labels, the vasculature. Imaging sessions are represented by the heading above images. Colored arrows indicate the X (red), Y (green), and Z (blue) directions. A–D: 0-wk1× (A), 2-wk1× (B), 4-wk1× (C), and 12-wk1× (D) images represent projected images of 200-μm deep stacks, taken at 40× magnification with 1× digital zoom, with a scale bar indicating 50 μm. White boxes denote the area that was imaged at 40× magnification with 3× digital zoom: 0-wk3× (E), 2-wk3× (F), 4-wk3× (G), and 12-wk3× (H) images show projection of 30-μm deep 3× stacks with a scale bar indicating 17 μm. I–K: 3-dimensional reconstructed mitochondria of image 0 wk3× (30 μm deep) from a side angle, in XY plane and in YZ perspective, respectively, with a sale bar indicating 10 μm.
Mitochondrial quality control.
The same image frames were selected from two major stacks, taken 30–60 min apart. The images were then deinterleaved and maximum projected. Images were compared side by side to demonstrate changes in mitochondrial signal (Fig. 6).
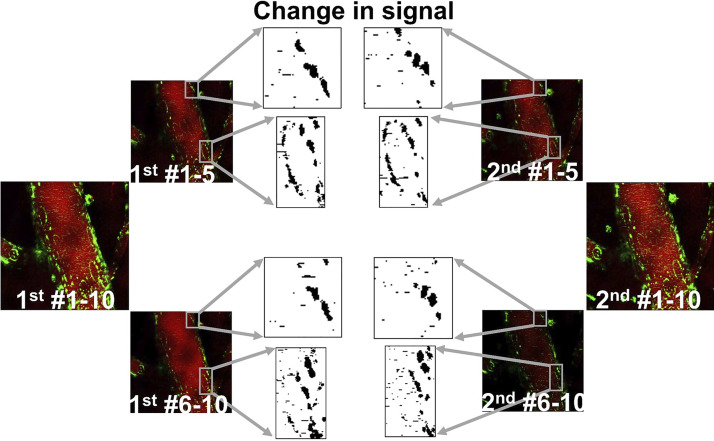
Fig. 6.
Dynamic changes in mitochondrial signal in large vessels. Time-lapse images of a large brain vessel show changes in mitochondrial signal. Red shows Rhodamine dextran-labeled plasma, whereas green shows endothelium-specific mito-Dendra2 signal. Zero or first time-point images are labeled as “1st #1–10.” Second set of images are noted as “2nd #1–10.” The numbers after the # sign are indicative of image frames. These images were split in half, resulting in the following subgroups: “1st #1–5” and “1st #6–10” and “2nd #1–5” and “2nd #6–10.” Gray boxes indicate the area with apparent changes in mitochondrial signal. Boxed area was enlarged and binarized images show changes in signal between the 2-imaging time points.
Statistics
Data were expressed as means (SD) and analyzed regions of interest of each group were indicated by n. One-way ANOVA with Kruskal-Wallis followed by Dunn’s test were used to test statistical significance using Graph Pad Prism 7.03. Values were statistically significant, if P < 0.05.
RESULTS AND DISCUSSION
We have developed a novel imaging method for determining mitochondrial characteristics in the in vivo cerebral endothelium in real time and repeatedly over several months in chronically instrumented mice.
Mice expressing mito-Dendra2 in the vascular endothelial mitochondria were used in our studies. Dendra is a monomeric variant of dendGFP that was first cloned by Labas et al. from Dendronephthya sp (17, 24), and excitation (λexc.490 nm) and emission (λem.507 nm) wavelengths for Dendra2 activation in its green state were reported by Chudakov et al. (5) Similarly, a 488-nm laser was used to excite mito-Dendra2 in fibroblast culture, vascular endothelial cells, as well as in muscle cells (10, 16, 31). While mito-Dendra2 is a fluorophore with photoconvertible properties (10, 16, 17, 24, 31), we did not employ those characteristics in our study. However, we propose that the combination of these properties with in vivo two-photon microscopy might shed light on the study of endothelial mitochondria in healthy and diseased brains in future studies. Mito-Dendra2 localization in the endothelium was confirmed in large brain vessels (Fig. 1, A and B) and aorta (Fig. 1, C and D), with a perinuclear distribution like that observed in the endothelium from fixed brain and cultured brain microvascular endothelial cells (Fig. 2). Mito-Dendra2 expression in the vascular endothelium of different-sized vascular beds were shown by us and others (10). Imaging or the dissection of these vessels can be used to determine mitochondrial content of large arteries compared with resistance arteries or capillaries. The Beyer group has demonstrated VE-cadherin-driven mito-Dendra2 expression of in vitro isolated and fixed arteries, which included the aorta and carotid, femoral, mesenteric, middle cerebral, and pulmonary arteries, as well as the jugular vein (without vessel-type quantification) (10). Thus, using these mice can provide valuable information about the mitochondrial content of veins, an understudied but important vessel type, and the role they might play in venous congestion-associated blood-brain-barrier disruption (13).
Our approach represents a major methodological advance compared with studies using mitochondrial-targeted fluorescent dyes that accumulate within the mitochondrion in a membrane potential-dependent fashion. The signal using other methods is not stable (e.g., environment and membrane potential dependent) and not specific to a particular cell type (14, 19, 32). In addition, our methodology is similar to those that use genetically encoded fluorescent probes expressed constitutively or in an inducible manner (1, 19, 25, 27). Combining high-resolution in vivo two-photon microscopy with genetically encoded mitochondrial labeling in the vascular endothelium allowed real-time determination and follow-up of temporal changes in mitochondria. Our approach will extend and complement other imaging techniques, such as those that determine membrane potential or reactive oxygen species production by mitochondria. Moreover, it overcomes difficulties arising from the repeated exogenous injection, distribution, or loading of mitochondrial dyes to label cerebrovascular endothelial mitochondria in vivo. In a recently published article by Durand et al. (10), the mito-Dendra2 signal was successfully used to determine mitochondrial structure under normal and high-glucose conditions in fixed vascular samples and cultured aortic endothelial cells. In our study, we demonstrate the feasibility of imaging mitochondria labeled via mito-Dendra2, repeatedly in the same animal, in the in vivo cerebral circulation under baseline conditions. Contrary to our expectations, based on studies of cultured endothelium, in vivo mitochondrial structures appeared fragmented rather than tubular as reported by others (10, 15, 23, 26). Morphological (length, tortuosity, and location within cell) and functional heterogeneity, as well as cell- and tissue-specific distribution and movement of mitochondria, have been reported among primary and transformed cell types (including human umbilical vein endothelial cells, cortical astrocytes, cortical neuronal cells, cardiomyocytes, and HeLa cells, porcine aortic endothelial cells, or COS‐7 cells, respectively) (6, 23).
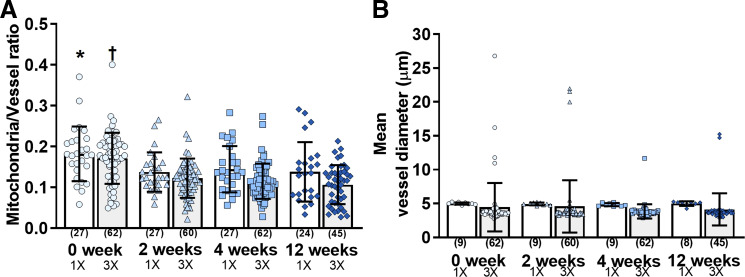
The 1× images (0 wk1×; 2 wk1×; 4 wk1×; and 12 wk1×; Fig. 4, A–D) provided an overview of the imaged vascular area, whereas the 3× images (0 wk3×; 2 wk3×; 4 wk3×; and 12 wk3×) revealed a greater resolution of a digitally zoomed vessel segment (Fig. 4, E–K). Mitochondrial and vessel area, as well as vessel diameters, were similar among the 1× and 3× groups (Fig. 5; Table 1). We found that the mean vessel diameter values ranged between 4.8 (SD 0.2) and 5.0 (SD 0.3) μm and 3.8 (SD 1.1) and 4.6 (SD 3.9) μm for 1× and 3× images, respectively, suggesting that the analyzed vasculature contained mainly capillaries (Fig. 5). The ratio determination of mitochondrial to vessel area revealed a significant difference (P < 0.05) only between 0 wk1× versus 12 wk1× and 0 wk3× versus 2–12 wk3×. Our 0-wk imaging session was performed 2 wk after the surgery, whereas the 2-wk imaging was done 4 wk after surgery. It may be that residual inflammation due to implantation of the window may have induced transient increases in mitochondrial dynamics, which subsided before the next viewing. However, chronic cranial window and similar imaging protocol (2 wk after surgery) have been used for studying structural and functional neuronal plasticity with mild and transient glial upregulation by several other laboratories (18). Contrarily, increased microglial and astrocyte activation were observed by Xu et al. (41) in open skull preparation (2 wk after surgery) when compared with thinned skull window or the contralateral hemisphere, which subsided approximately 4 wk following open skull surgery. Value stability over the next three imaging sessions negated any appreciable bleaching effect of the mito-Dendra2 signal. Altogether, based on our results, the mitochondrial signal was quantifiable and can detect even minor changes in total mito-Dendra2 signal. Furthermore, we propose that our imaging approach will enable the detection and quantification of mitochondrial movement as well as fission/fusion processes in vivo. Continuous fission and fusion events, at a rate of ~65 events/min for 103 objects, as well as Brownian motion, resulting in more than 0.85-μm traveled distance of ~28% of objects compared with their original position in control cells, have been reported by Giedt et al. (15) in HUVECs. In this study, we demonstrated that changes in mitochondrial signal can be identified in endothelial mitochondria using two-photon imaging, but further study is needed to provide quantification of these processes (Fig. 6). A potential limitation of our study was that we included the use of the selected animal strains to label endothelial cells and mitochondria rather than other models of the Cre/Lox system that are available. While we used a relatively short 12-wk follow-up, future studies can be extended to up to 12 mo (28).
Fig. 5.
Quantification of mitochondrial and vascular signal. A: mitochondrial and vessel area ratios were similar among groups throughout the 4 consecutive imaging sessions using 1× (0 wk1×, 2 wk1×, 4 wk1×, and 12 wk1×) and 3× (0 wk3×, 2 wk3×, 4 wk3×, and 12 wk3×) digital zoom, except for 0 wk1x vs. 12 wk1x (*P < 0.05) and 0 wk3x vs. 2–12 wk3x (†P < 0.05). B: mean vessel diameter was similar among groups. Data were expressed as means (SD), and numbers in parenthesis indicate quantified areas.
Table 1.
Quantification of mitochondria and vasculature
| Groups |
Mean Vessel Diameter, μm |
|||||
|---|---|---|---|---|---|---|
| n | Mitochondrial Area, pixel | Vessel Area, pixel | Mitochondrial-to-Vessel Ratio | n | ||
| 1× | ||||||
| 0 wk1× | 27 | 5,966 (SD 4,800) | 30,493 (SD 14,757) | 0.182 (SD 0.067)* | 5.00 (SD 0.2) | 9 |
| 2 wk1× | 27 | 4,830 (SD 5,036) | 31,416 (SD 23,943) | 0.138 (SD 0.048) | 4.95 (SD 0.2) | 9 |
| 4 wk1× | 27 | 4,108 (SD 3,619) | 27,650 (SD 16,423) | 0.144 (SD 0.057) | 4.84 (SD 0.2) | 9 |
| 12 wk1× | 24 | 6,005 (SD 5,812) | 41,675 (SD 27,219) | 0.138 (SD 0.073) | 5.00 (SD 0.3) | 8 |
| 3× | ||||||
| 0 wk3× | 62 | 5,476 (SD 2,456)‡ | 33,583 (SD 13,160) | 0.171 (SD 0.062)† | 4.46 (SD 3.6) | 62 |
| 2 wk3× | 60 | 3,917 (SD 1,773) | 33,811 (SD 15,691) | 0.123 (SD 0.048) | 4.58 (SD 3.9) | 60 |
| 4 wk3× | 62 | 3,353 (SD 2,123) | 30,210 (SD 15,793)§ | 0.115 (SD 0.043) | 3.84 (SD 1.1) | 62 |
| 12 wk3× | 45 | 3,447 (SD 1,309) | 36,836 (SD 17,117) | 0.107 (SD 0.048) | 4.14 (SD 2.4) | 45 |
Values are means (SD). Area measurements for mitochondria and vessels area provided in pixel. Total image area (A) at 1× and 3× is equal to 262,144 pixel, and A1× = 138,736 μm2 and A3× = 15,813 μm2. Mitochondrial-to-vessel ratio was calculated based on the area values expressed in pixel.
P < 0.05, mitochondrial-to-vessel ratio 0 wk1x vs. 12 wk1x;
P < 0.05, mitochondrial-to-vessel ratio 0 wk3x vs. 2–12 wk3x;
P < 0.05, mitochondrial area 0 wk3x vs. 2–12 wk3x; and
P = 0.0484. Vessel area 4 wk3x vs. 12 wk3x.
In conclusion, our novel approach can be used to study real-time mitochondrial processes under normal conditions and during aging (36, 38, 39) to investigate the role of mitochondria in neurovascular coupling (7, 42) or in diseases including diabetes (10), hypertension (8), Alzheimer’s disease (4), or cognitive impairment (37).
GRANTS
This work was supported in part by National Institutes of Health Grants U54-GM-0104940, which funds the Louisiana Clinical and Translational Science Center (to I. Rutkai); NS-094834 (to P. V. G. Katakam); HL-077731, HL093554, and HL-148836 (to D. W. Busija); AG-063345 (to D. W. Busija); and R01-AG-047296 (to R. Mostany); by American Heart Association Scientist Development Grants 17SDG33410366 (to I. Rutkai) and 14SDG20490359 (to P. V. G. Katakam); by Louisiana Board of Regents Grants, e.g., Endowed Chairs for Eminent Scholars program (to D. W. Busija) and Research Competitiveness Subprogram from the Board of Regents Support Fund R&D Program LEQSF(2016-19)-RD-A-24 (to R. Mostany); and National Science Foundation Office of Integrative Activities’ Established Program to Stimulate Competitive Research Section Grant 1539067 (to R. Mostany).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.R., W.R.E., and D.W.B. conceived and designed research; I.R., W.R.E., N.B., T.S.-C., and S.C. performed experiments; I.R., N.B., T.S.-C., and S.C. analyzed data; I.R. and D.W.B. interpreted results of experiments; I.R. and N.B. prepared figures; I.R. drafted manuscript; I.R., W.R.E., N.B., T.S.-C., S.C., P.K.C., P.V.G.K., R.M., and D.W.B. edited and revised manuscript; I.R., W.R.E., N.B., T.S.-C., S.C., P.K.C., P.V.G.K., R.M., and D.W.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ashwin Adivi, Tyler Dean, and Dan Liu for technical help; Nancy Busija for manuscript editing; LACaTS Center support; and Timothy Tetreault and MBF Bioscience for help with image reconstruction.
REFERENCES
- 1.Agarwal A, Wu PH, Hughes EG, Fukaya M, Tischfield MA, Langseth AJ, Wirtz D, Bergles DE. Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 93: 587–605, 2017. doi: 10.1016/j.neuron.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berman SB, Pineda FJ, Hardwick JM. Mitochondrial fission and fusion dynamics: the long and short of it. Cell Death Differ 15: 1147–1152, 2008. doi: 10.1038/cdd.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosetti F, Galis ZS, Bynoe MS, Charette M, Cipolla MJ, Del Zoppo GJ, Gould D, Hatsukami TS, Jones TL, Koenig JI, Lutty GA, Maric-Bilkan C, Stevens T, Tolunay HE, Koroshetz W; Small Blood Vessels: Big Health Problems” Workshop Participants . “Small blood vessels: big health problems?”: scientific recommendations of the National Institutes of Health workshop. J Am Heart Assoc 5: e004389, 2016. doi: 10.1161/JAHA.116.004389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cenini G, Voos W. Mitochondria as potential targets in Alzheimer disease therapy: an update. Front Pharmacol 10: 902, 2019. doi: 10.3389/fphar.2019.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chudakov DM, Lukyanov S, Lukyanov KA. Tracking intracellular protein movements using photoswitchable fluorescent proteins PS-CFP2 and Dendra2. Nat Protoc 2: 2024–2032, 2007. doi: 10.1038/nprot.2007.291. [DOI] [PubMed] [Google Scholar]
- 6.Collins TJ, Berridge MJ, Lipp P, Bootman MD. Mitochondria are morphologically and functionally heterogeneous within cells. EMBO J 21: 1616–1627, 2002. doi: 10.1093/emboj/21.7.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csiszar A, Yabluchanskiy A, Ungvari A, Ungvari Z, Tarantini S. Overexpression of catalase targeted to mitochondria improves neurovascular coupling responses in aged mice. Geroscience 41: 609–617, 2019. doi: 10.1007/s11357-019-00111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czigler A, Toth L, Szarka N, Berta G, Amrein K, Czeiter E, Lendvai-Emmert D, Bodo K, Tarantini S, Koller A, Ungvari Z, Buki A, Toth P. Hypertension Exacerbates Cerebrovascular Oxidative Stress Induced by Mild Traumatic Brain Injury: Protective Effects of the Mitochondria-Targeted Antioxidative Peptide SS-31. J Neurotrauma 36: 3309–3315, 2019. doi: 10.1089/neu.2019.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Bock K, Georgiadou M, Carmeliet P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab 18: 634–647, 2013. doi: 10.1016/j.cmet.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Durand MJ, Ait-Aissa K, Levchenko V, Staruschenko A, Gutterman DD, Beyer AM. Visualization and quantification of mitochondrial structure in the endothelium of intact arteries. Cardiovasc Res 115: 1546–1556, 2019. doi: 10.1093/cvr/cvy294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eelen G, de Zeeuw P, Treps L, Harjes U, Wong BW, Carmeliet P. Endothelial cell metabolism. Physiol Rev 98: 3–58, 2018. doi: 10.1152/physrev.00001.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferree A, Shirihai O. Mitochondrial dynamics: the intersection of form and function. Adv Exp Med Biol 748: 13–40, 2012. doi: 10.1007/978-1-4614-3573-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fulop GA, Ahire C, Csipo T, Tarantini S, Kiss T, Balasubramanian P, Yabluchanskiy A, Farkas E, Toth A, Nyúl-Tóth Á, Toth P, Csiszar A, Ungvari Z. Cerebral venous congestion promotes blood-brain barrier disruption and neuroinflammation, impairing cognitive function in mice. Geroscience 41: 575–589, 2019. doi: 10.1007/s11357-019-00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerencser AA, Chinopoulos C, Birket MJ, Jastroch M, Vitelli C, Nicholls DG, Brand MD. Quantitative measurement of mitochondrial membrane potential in cultured cells: calcium-induced de- and hyperpolarization of neuronal mitochondria. J Physiol 590: 2845–2871, 2012. doi: 10.1113/jphysiol.2012.228387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giedt RJ, Pfeiffer DR, Matzavinos A, Kao CY, Alevriadou BR. Mitochondrial dynamics and motility inside living vascular endothelial cells: role of bioenergetics. Ann Biomed Eng 40: 1903–1916, 2012. doi: 10.1007/s10439-012-0568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glancy B, Hartnell LM, Combs CA, Femnou A, Sun J, Murphy E, Subramaniam S, Balaban RS. Power Grid Protection of the Muscle Mitochondrial Reticulum. Cell Reports 19: 487–496, 2017. doi: 10.1016/j.celrep.2017.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurskaya NG, Verkhusha VV, Shcheglov AS, Staroverov DB, Chepurnykh TV, Fradkov AF, Lukyanov S, Lukyanov KA. Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat Biotechnol 24: 461–465, 2006. doi: 10.1038/nbt1191. [DOI] [PubMed] [Google Scholar]
- 18.Holtmaat A, Bonhoeffer T, Chow DK, Chuckowree J, De Paola V, Hofer SB, Hübener M, Keck T, Knott G, Lee WC, Mostany R, Mrsic-Flogel TD, Nedivi E, Portera-Cailliau C, Svoboda K, Trachtenberg JT, Wilbrecht L. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protoc 4: 1128–1144, 2009. doi: 10.1038/nprot.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakobs S. High resolution imaging of live mitochondria. Biochim Biophys Acta 1763: 561–575, 2006. doi: 10.1016/j.bbamcr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Xiao J, Wan J, Cohen P, Yen K. Mitochondrially derived peptides as novel regulators of metabolism. J Physiol 595: 6613–6621, 2017. doi: 10.1113/JP274472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol 230: 230–242, 2001. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 22.Kluge MA, Fetterman JL, Vita JA. Mitochondria and endothelial function. Circ Res 112: 1171–1188, 2013. doi: 10.1161/CIRCRESAHA.111.300233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuznetsov AV, Hermann M, Saks V, Hengster P, Margreiter R. The cell-type specificity of mitochondrial dynamics. Int J Biochem Cell Biol 41: 1928–1939, 2009. doi: 10.1016/j.biocel.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Labas YA, Gurskaya NG, Yanushevich YG, Fradkov AF, Lukyanov KA, Lukyanov SA, Matz MV. Diversity and evolution of the green fluorescent protein family. Proc Natl Acad Sci USA 99: 4256–4261, 2002. doi: 10.1073/pnas.062552299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lippincott-Schwartz J, Patterson GH. Photoactivatable fluorescent proteins for diffraction-limited and super-resolution imaging. Trends Cell Biol 19: 555–565, 2009. doi: 10.1016/j.tcb.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarron JG, Wilson C, Sandison ME, Olson ML, Girkin JM, Saunter C, Chalmers S. From structure to function: mitochondrial morphology, motion and shaping in vascular smooth muscle. J Vasc Res 50: 357–371, 2013. doi: 10.1159/000353883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitra K, Lippincott-Schwartz J. Analysis of mitochondrial dynamics and functions using imaging approaches. Curr Protoc Cell Biol. Chapter 4: Unit 4.25.1–21, 2010. doi: 10.1002/0471143030.cb0425s46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mostany R, Anstey JE, Crump KL, Maco B, Knott G, Portera-Cailliau C. Altered synaptic dynamics during normal brain aging. J Neurosci 33: 4094–4104, 2013. doi: 10.1523/JNEUROSCI.4825-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mostany R, Portera-Cailliau C. A craniotomy surgery procedure for chronic brain imaging. J Vis Exp 12: 680, 2008. doi: 10.3791/680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ollion J, Cochennec J, Loll F, Escudé C, Boudier T. TANGO: a generic tool for high-throughput 3D image analysis for studying nuclear organization. Bioinformatics 29: 1840–1841, 2013. doi: 10.1093/bioinformatics/btt276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pham AH, McCaffery JM, Chan DC. Mouse lines with photo-activatable mitochondria to study mitochondrial dynamics. Genesis 50: 833–843, 2012. doi: 10.1002/dvg.22050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poot M, Zhang YZ, Krämer JA, Wells KS, Jones LJ, Hanzel DK, Lugade AG, Singer VL, Haugland RP. Analysis of mitochondrial morphology and function with novel fixable fluorescent stains. J Histochem Cytochem 44: 1363–1372, 1996. doi: 10.1177/44.12.8985128. [DOI] [PubMed] [Google Scholar]
- 33.Ruck T, Bittner S, Epping L, Herrmann AM, Meuth SG. Isolation of primary murine brain microvascular endothelial cells. J Vis Exp 93: e52204, 2014. doi: 10.3791/52204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sure VN, Sakamuri SSVP, Sperling JA, Evans WR, Merdzo I, Mostany R, Murfee WL, Busija DW, Katakam PVG. A novel high-throughput assay for respiration in isolated brain microvessels reveals impaired mitochondrial function in the aged mice. Geroscience 40: 365–375, 2018. doi: 10.1007/s11357-018-0037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell 17: e12731, 2018. doi: 10.1111/acel.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol 294: H2121–H2128, 2008. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- 39.Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of Vascular Aging. Circ Res 123: 849–867, 2018. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wikstrom JD, Twig G, Shirihai OS. What can mitochondrial heterogeneity tell us about mitochondrial dynamics and autophagy? Int J Biochem Cell Biol 41: 1914–1927, 2009. doi: 10.1016/j.biocel.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Xu HT, Pan F, Yang G, Gan WB. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat Neurosci 10: 549–551, 2007. doi: 10.1038/nn1883. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 163: 1064–1078, 2015. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]