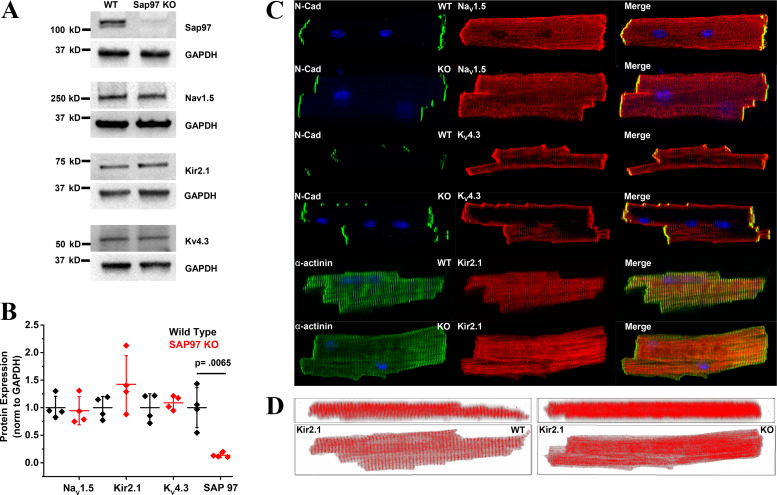
Fig. 2.
Knockdown of synapse-associated protein 97 (Sap97) alters subcellular distribution of Kir2.1. A: Western blots of Sap97, NaV1.5, KV4.3, and Kir2.1 in heart lysates from wild-type (WT) and Sap 97-knockout (KO) animals (N = 4,4). B: quantification of the Western blots demonstrates that downregulation of Sap97 (*P = 0.0065) in Sap 97-KO animals does not alter protein profiles of the major cardiac ion channels. Horizontal bars represent means; vertical bars, SD. C: confocal images of ventricular myocytes isolated from adult WT and Sap 97-KO mice. NaV1.5 and Kv4.3 protein retain their subcellular distribution. Subcellular organization of Kir2.1 protein is significantly disrupted in KO cells. D: confocal z-stacks represented in two dimensions illustrate that the striated organization of Kir2.1 is lost in Sap97-KO cells. N-cadherin (N-Cad) is used as a marker for intercalated disk and α-actinin for sarcomeric structure.