Abstract
Background
Although people living with human immunodeficiency virus (PLWH) are at increased risk of invasive pneumococcal disease (IPD) and community-acquired pneumonia (CAP), it is unclear whether this remains the case in the setting of early initiation of combination antiretroviral therapy (cART), at high CD4 cell counts. This is important, as pneumococcal vaccination coverage in PLWH is low in Europe and the United States, despite longstanding international recommendations.
Methods
We identified all CAP and IPD cases between 2008 and 2017 in a cohort of PLWH in a Dutch HIV referral center. We calculated incidence rates stratified by CD4 count and cART status and conducted a case-control study to identify risk factors for CAP in PLWH receiving cART.
Results
Incidence rates of IPD and CAP in PLWH were 111 and 1529 per 100 000 patient-years of follow-up (PYFU). Although IPD and CAP occurred more frequently in patients with CD4 counts <500 cells/μL (incidence rate ratio [IRR], 6.1 [95% confidence interval, 2.2–17] and IRR, 2.4 [95% confidence interval, 1.9–3.0]), the incidence rate in patients with CD4 counts >500 cells/μL remained higher compared with the general population (946 vs 188 per 100 000 PYFU). All IPD isolates were vaccine serotypes. Risk factors for CAP were older age, CD4 counts <500 cells/μL, smoking, drug use, and chronic obstructive pulmonary disease.
Conclusions
The incidence of IPD and CAP among PLWH remains higher compared with the general population, even in those who are virally suppressed and have high CD4 counts. With all serotyped IPD isolates covered by pneumococcal vaccines, our study provides additional argumentation against the poor current adherence to international recommendations to vaccinate PLWH.
Keywords: HIV, pneumococcal disease, community-acquired pneumonia, epidemiology, vaccination
The incidence of pneumococcal disease and pneumonia among people living with HIV (PLWH) remains higher compared with the general population, even in those who are virally suppressed, and have high CD4 cell counts. Pneumococcal vaccination should be considered in all PLWH.
(See the Editorial Commentary by Jenny-Avital on pages 51–2.)
People living with human immunodeficiency virus (PLWH) are at increased risk of both invasive pneumococcal disease (IPD) and community-acquired pneumonia (CAP) caused by Streptococcus pneumoniae [1]. Pneumococcal disease (PD; ie, IPD and pneumococcal CAP), in PLWH often requires hospitalization, and mortality rates range up to 25% [2, 3]. In addition, the recurrence rate of PD is high [4]. Therefore, PD remains an important problem in PLWH, contributing significantly to disease burden and healthcare costs [5, 6]. Pneumococcal vaccination is recommended for all PLWH by most international guidelines [7–9]. However, despite longstanding recommendations, pneumococcal vaccine uptake is low [5, 10–12]. This is worrying, as a recent meta-analysis showed that the incidence rate of IPD remains approximately 30 times higher among PLWH compared to the general population in the modern era of combination antiretroviral therapy (cART)—that is, in the period from 2000 onward, when effective cART was available [2]. An oft-mentioned argument against pneumococcal vaccination in all PLWH is that cases of PD represent an untreated or severely immunocompromised subgroup [13]. Unfortunately, most individual studies on the incidence of PD in the late cART era are based on bacterial surveillance data and lack clinical information on CD4 cell counts, cART use, viral load, and other risk factors [2, 14]. In addition, many of these study data originate from before 2015, when early start of cART, irrespective of CD4 count and viral load, became the cornerstone of human immunodeficiency virus (HIV) care [15, 16].
Thus, it is yet unclear whether the risk of PD in the growing group of PLWH with high CD4 counts who started cART shortly after their HIV diagnosis has declined to the level of the non-PLWH, or whether it is still substantially increased, which may provide more compelling evidence for the recommendation of universal vaccination in PLWH.
The purpose of this cohort study was to determine the incidence of IPD and CAP in PLWH stratified to CD4 cell count and treatment status, between 2008 and 2017. Additionally, we aimed to identify risk factors for pneumococcal disease in PLWH.
METHODS
Cohort Study
We identified all IPD and CAP cases from June 2008 until December 2017 in PLWH who received care at the Amsterdam University Medical Center (UMC), location Academic Medical Centers (AMCs). Within this cohort, we calculated incidence rates of CAP and IPD stratified by CD4 cell count and cART status.
IPD Case Definition and Identification
We defined a case of IPD as a culture-proven (blood or cerebrospinal fluid) infection with S. pneumoniae. All IPD cases occurring in the Amsterdam UMC-AMC are reported to the Netherlands Reference Laboratory for Bacterial Meningitis (NRLBM) as part of a national pneumococcal surveillance system [14]. For the years 2008–2016, the HIV status of all IPD cases reported to the NRLBM was collected as part of another study [14] and Vestjens et al, unpublished data). We appended data for 2017 to this dataset.
We counted infections within 30 days in the same patient as a single case. The NRLBM used co-agglutination and the Quellung method for serotyping of pneumococcal isolates from IPD cases [17].
CAP Case Definition and Identification
The Dutch HIV Monitoring Foundation (Stichting HIV Monitoring [SHM]) prospectively collects data of PLWH in the Netherlands (the AIDS Therapy Evaluation in the NetherlAnds [ATHENA] cohort). The ATHENA cohort was initiated in 1998 and captures data, including cART use, HIV type 1 viral loads, CD4 cell counts, and opportunistic infections, from >98% of all PLWH in the Netherlands [18, 19]. An episode of CAP is registered in the ATHENA database if it is mentioned in the electronic patient file. The SHM provided us with all registered cases of CAP in PLWH in care of the Amsterdam UMC-AMC between June 2008 and December 2017.
For all registered ATHENA cases of CAP, we reviewed the electronic patient file. A case of CAP was confirmed and included in our study if it was community-acquired (>60 days after discharge if a patient was previously admitted to a hospital), diagnosed on clinical, microbiological, and/or radiological grounds, and required antibiotic treatment.
Based on the microbiologic results, we divided all CAP cases into 3 subgroups: (1) pneumococcal CAP, if there was at least 1 positive culture for S. pneumoniae in sputum and/or blood; (2) “unspecified CAP,” when no pathogen was cultured; (3) “other pathogen (OP) CAP,” when we found documentation of 1 or more cultured pathogen(s) other than S. pneumoniae.
Case-control Study
We performed a nested case-control study to identify risk factors for pneumococcal and unspecified CAP in PLWH receiving cART. For each case, 1 control matched for year of HIV diagnosis was selected. Controls were not matched for cART use to avoid overmatching. For the case-control study, we excluded CAP cases in which another pathogen was cultured (OP), as this subgroup’s complexity was beyond the scope of our study. Each control had to be in care at the Amsterdam UMC-AMC in the year the linked case was diagnosed with CAP.
Data Extraction and Analysis
We collected clinical and demographical data of all cases and controls from the electronic patient file and online vaccination registry (Orion Globe, Assen, the Netherlands) using a standardized case report form. Data items collected were date of HIV diagnosis; ethnicity; mode of HIV acquisition; viral load; CD4 and nadir CD4 cell counts; cART use; comorbidities; smoking, alcohol, or drug use; pneumococcal vaccination status and vaccination date; mortality; and duration of hospital admission.
Longitudinal data collected by SHM on all PLWH in care in the Amsterdam UMC-AMC were used to calculate cumulative patient-years of follow-up (PYFU) from 1 June 2008 until 31 December 2017. HIV PYFU was stratified by calendar year, CD4 cell count, and treatment status.
We calculated the incidence rates (IRs) of IPD, pneumococcal CAP, PD (pneumococcal CAP + IPD), and all CAP in total and per subgroup, based on CD4 count (<500 cells/μL and ≥500 cells/μL), cART treatment status, and the occurrence of the disease before or after 2015. If an IPD episode originated from CAP, this case was only counted once in incidence calculations for pneumococcal disease. We calculated incidence rate ratios (IRRs) per subgroup with 95% confidence intervals (CIs).
For the incidence calculations, we excluded patients with a newly diagnosed HIV infection, defined as date of HIV diagnosis within 60 days of the IPD or CAP episode, because the IPD or CAP episode in these cases was often the trigger for establishing the HIV diagnosis, and could thus not have been prevented by vaccination.
For statistical analyses, we used SPSS statistical software, version 25 (IBM SPSS, Armonk, New York). We applied an α level = .05 for significance. We performed multivariable conditional regression analysis to identify risk factors for pneumococcal/unspecified CAP in PLWH receiving cART. In our initial model, we included the covariates age, sex, mode of HIV acquisition, viral load, CD4 cell count subgroups, smoking, alcohol, chronic obstructive pulmonary disease (COPD), kidney diseases, cardiovascular diseases, asthma, drug use, malignancies, and hepatitis B and C coinfection. Covariates were excluded from the model using the stepwise backward selection method based on P value <.05. We performed a subgroup analysis excluding cART-discordant case-control pairs to assess if this would change the effect size of our results.
Ethical Considerations
For this noninterventional study, an exemption was granted by the local medical ethics committee on 29 June 2018 (W18_204; document with the authors).
RESULTS
From June 2008 to December 2017, during 18 898 PYFU, we observed 24 cases of IPD in 21 PLWH and 318 episodes of CAP in 215 PLWH. In 18 of 24 (75%) IPD cases, patients initially presented with the clinical diagnosis of CAP, and were consequently classified as having bacteremic pneumococcal CAP, resulting in a total of 324 episodes of IPD and/or CAP.
Of all 318 cases of CAP, 36 (11%) were culture-proven pneumococcal infections; in 214 (67%) cases, no pathogen was identified (unspecified CAP); and in 68 cases (21%), a different pathogen was identified (OP CAP). The case fatality rates were 8.3% and 3.5% for IPD and CAP, respectively. Baseline characteristics of all cases are shown in Table 1. Baseline characteristics differed between the different CAP subgroups. Compared with pneumococcal CAP and unspecified CAP, the OP CAP group was the most severely immunocompromised, with the lowest median and nadir CD4 counts, the fewest patients on cART, the fewest patients with an undetectable viral load, and the highest proportion of patients with comorbidities. The majority of cases in the OP group (54%) met the criteria of AIDS at the time of CAP diagnosis, and 15 of 70 (22%) were newly diagnosed with HIV (Table 1).
Table 1.
Baseline Characteristics of All Included Episodes of Invasive Pneumococcal Disease and Community-acquired Pneumonia
| Characteristic | All Cases (N = 324) | IPD | CAP | P Valuea | ||||
|---|---|---|---|---|---|---|---|---|
| All CAP | Pneumococcal CAP | Undefined Pathogen CAP | Other Pathogen CAP | IPD Compared to CAP Without IPD | CAP Subgroups Compared | |||
| (n = 24) | (n = 318) | (n = 36) | (n = 214) | (n = 68) | ||||
| Male sex | 222/324 (68) | 15/24 (63) | 218/318 (69) | 23/36 (64) | 154/214 (72) | 41/68 (60) | .51 | .16 |
| Age, y, median (IQR) | 50 (41–60) | 41 (29–55) | 50 (41–60) | 43 (35–53) | 51 (43–60) | 50 (39–61) | .01 | <.01 |
| Mode of HIV acquisition | .35 | .08 | ||||||
| Sex (heterosexual) | 122/299 (41) | 10/23 (44) | 122/293 (42) | 20/34 (59) | 74/200 (37) | 28/59 (48) | ||
| Intravenous drug use | 30/299 (10) | 3/23 (13) | 30/293 (10) | 5/34 (15) | 17/200(9) | 8/59 (14) | ||
| MSM | 137/299(46) | 8/23 (35) | 133/293 (45) | 9/34 (27) | 101/200 (51) | 23/59 (39) | ||
| Mother to child | 8/299 (2.7) | 2/23 (8.7) | 6/293 (2.0) | 0/34 (0) | 6/200 (3) | 0/59 (0) | ||
| Blood transfusion | 2/299 (0.7) | 0/23 (0) | 2/293 (0.7) | 0/34 (0) | 2/200 (1) | 0/59 (0) | ||
| Ethnic group | .15 | .16 | ||||||
| White European | 189/315 (60) | 10/22 (46) | 187/310 (60) | 19/35 (54) | 132/209 (63) | 36/66 (55) | ||
| Asian | 12/315 (3.8) | 0/22 (0) | 12/310 (3.9) | 0/35 (0) | 9/209 (4.3) | 3/66 (4.5) | ||
| Black African | 93/315 (30) | 11/22 (44) | 90/310 (29) | 15/35 (43) | 51/209 (25) | 24/66 (36) | ||
| Hispanic | 21/315 (6.7) | 1/22 (4.5) | 21/310 (6.8) | 1/35 (2.9) | 17/209 (8.2) | 3/66 (4.5) | ||
| Nadir CD4 count, No; median (IQR) | 321; 180 (70–290) | 24; 265 (115–403) | 315; 180 (70–290) | 36; 220 (103–347) | 211; 180 (80–300) | 68; 145 (33–227) | .03 | .02 |
| Date of diagnosis | .16 | .07 | ||||||
| Before 1 January 2015 | 229/324 (71) | 20/24 (83) | 224/318 (70) | 29/36 (81) | 142/214 (66) | 53/68 (78) | ||
| After 1 January 2015 | 95/324 (29) | 4/24 (17) | 94/318 (30) | 7/38 (19) | 72/214 (34) | 15/68 (22) | ||
| Newly diagnosed HIV infectionb | 29/324 (9) | 3/24 (13) | 29/318 (9.1) | 6/36 (17) | 8/214 (3.7) | 15/68 (22) | .53 | <.01 |
| Duration of HIV, mo, median (IQR) | 120 (49–215) | 63 (9–124) | 121 (49–217) | 76 (9–196) | 143 (60–221) | 90 (7–175) | .02 | <.01 |
| CD4 count at time of diagnosis, median (IQR) | 440 (220–670) | 345 (235–470) | 445 (220–670) | 345 (240–478) | 490 (300–715) | 290 (40–532) | .18 | <.01 |
| CD4 count at time of diagnosis | .03 | <.01 | ||||||
| <200 cells/μL | 70/324 (22) | 4/24 (17) | 68/318 (21) | 6/36 (17) | 36/214 (17) | 26/68 (38) | ||
| 200–499 cells/μL | 123/324 (38) | 15/24 (63) | 121/318 (38) | 22/36 (61) | 75/214 (35) | 24/68 (35) | ||
| ≥500 cells/μL | 131/324 (40) | 5/24 (21) | 129/318 (41) | 8/36 (22) | 103/214 (48) | 18/68 (27) | ||
| Undetectable viral load at time of diagnosis | 193/324 (60) | 10/24 (41) | 188/318 (59) | 14/36 (39) | 148/213 (69) | 26/68 (38) | .06 | <.01 |
| AIDS at time of diagnosis | 86/334 (27) | 6/24 (25) | 84/318 (26) | 9/36 (25) | 38/214 (18) | 37/68 (54) | .86 | <.01 |
| cART at time of diagnosis | 224/324 (69) | 14/24 (58) | 218/315 (69) | 20/36 (56) | 168/213 (79) | 30/66 (46) | .39 | <.01 |
| Duration of cART, mo, No.; median (IQR) | 224; 102 (32–175) | 14; 54 (19–144) | 218; 102 (32–178) | 20; 68 (29–166) | 168; 106 (32–185) | 30; 103 (42–165) | .17 | .48 |
| Hospitalization | 197/316 (62) | 17/24 (71) | 194/311 (62) | 29/35 (83) | 106/209 (51) | 59/67 (88) | .37 | <.01 |
| Duration of hospitalization, d, No.; median (IQR) | 168; 7 (4–12) | 15; 7 (4–21) | 166; 7 (4–12) | 25; 6 (5–12) | 84; 6 (4–10) | 57; 10 (5–18) | .54 | .03 |
| ICU admission | 29/292 (9.9) | 7/22 (32) | 29/288 (10) | 8/38 (25) | 8/191 (4.2) | 13/65 (20) | <.01 | <.01 |
| Disease-related mortalityc | 11/324 (3.4) | 2/24 (8.3) | 11/318 (3.5) | 2/36 (5.6) | 4/214 (1.9) | 5/68 (7.4) | .19 | .08 |
| Comorbidities at time of diagnosis | ||||||||
| Any comorbidity | 210/324 (65) | 14/24 (58) | 205/318 (65) | 19/36 (53) | 134/214 (63) | 52/68 (77) | .49 | .03 |
| Cardiovascular diseased | 73/324 (23) | 3/24 (13) | 73/318 (23) | 3/36 (8.3) | 50/214 (23) | 20/68 (29) | .22 | .05 |
| COPD | 85/324 (26) | 1/24 (4.2) | 85/318 (27) | 9/36 (25) | 56/214 (26) | 20/68 (29) | .01 | .84 |
| Asthma | 15/324 (4.6) | 0/24 (0) | 15/318 (4.7) | 2/36 (5.6) | 7/214 (3.3) | 6/68 (8.8) | .26 | .17 |
| Diabetes | 32/324 (9.9) | 1/24 (4.2) | 32/318 (10) | 3/36 (8.3) | 14/214 (6.5) | 15/68 (22) | .49 | <.01 |
| Asplenia | 11/324 (3.4) | 4/24 (17) | 9/318 (2.8) | 2/34 (5.6) | 6/214 (2.8) | 1/68 (1.5) | .01 | .49 |
| Malignancy | 35/324 (11) | 4/24 (17) | 32/318 (10) | 1/36 (2.8) | 26/214 (12) | 5/68 (7.4) | .55 | .16 |
| Chronic kidney disease | 38/324 (12) | 7/24 (30) | 35/318 (11) | 4/36 (11) | 22/214 (10) | 9/68 (13) | .01 | .79 |
| Liver cirrhosis | 11/324 (3.4) | 3/24 (13) | 9/318 (2.8) | 1/36 (2.8) | 6/214 (2.8) | 2/68 (2.9) | .04 | 1.00 |
| Other immunodeficiency disordere | 15/324 (4.6) | 4/24 (17) | 12/318 (3.8) | 1/36 (2.8) | 10/214 (4.7) | 1/68 (1.5) | .02 | .46 |
| Hepatitis Cf | 34/324 (11) | 3/24 (13) | 34/318 (11) | 5/36 (14) | 22/214 (10) | 7/68 (10) | .74 | .81 |
| Hepatitis Bg | 18/324 (5.6) | 1/24 (4.2) | 18/318 (5.7) | 1/36 (2.8) | 11/214 (5.1) | 6/68 (8.8) | .76 | .38 |
| Smoking at time of diagnosis | 169/298 (57) | 10/22 (46) | 169/292 (58) | 19/31 (61) | 117/202 (58) | 33/59 (56) | .27 | .89 |
| Alcohol use at time of diagnosis | 189/282 (67) | 16/20 (80) | 185/276 (67) | 23/29 (79) | 125/190 (66) | 37/57 (65) | .20 | .33 |
| Drug use at time of diagnosish | 88/265 (33) | 6/20 (30) | 88/259 (34) | 13/29 (45) | 60/175 (34) | 15/55 (27) | .75 | .27 |
| Pneumococcal vaccination prior to diagnosisi | 19/324 (5.9) | 2/24 (8.3) | 19/318 (6.0) | 5/36 (14) | 12/214 (5.6) | 2/68 (2.9) | .64 | .08 |
Data are presented as no./No. (%) unless otherwise indicated.
Abbreviations: CAP, community-acquired pneumonia; cART, combination antiretroviral therapy; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; ICU, intensive care unit; IgG, immunoglobulin G; IPD, invasive pneumococcal disease; IQR, interquartile range; MSM, men who have sex with men.
aBold text indicates significant difference between subgroups (P < .05).
bWithin 60 days after diagnosis.
cWithin 30 days after diagnosis.
dAll types of cardiovascular disease including hypertension, (ischemic) cardiomyopathy, coronary artery disease, any type of valve disease, arrhythmias, and (chronic) heart failure.
eLeukemia, lymphoma, kidney transplant, IgG subclass deficiency, use of immunosuppressive drugs for inflammatory diseases.
fChronic hepatitis C infection.
gActive hepatitis B infection or inactive carrier.
hAny type of drug use including cannabis, cocaine, heroin, methamphetamine, amyl nitrate (“poppers”), ecstasy, γ-hydroxybutyrate, ketamine, and methadone.
iAdministration of any pneumococcal vaccine prior to diagnosis. Five of 324 cases were vaccinated with both Prevenar13 and Pneumovax23 according to international guidelines.
Incidence Rates
The overall IRs of IPD, CAP, and pneumococcal CAP were 111, 1529, and 159 per 100 000 PYFU, respectively. We found no differences in IRs before and after 1 January 2015 for CAP, pneumococcal CAP, and IPD (Supplementary Table 1).
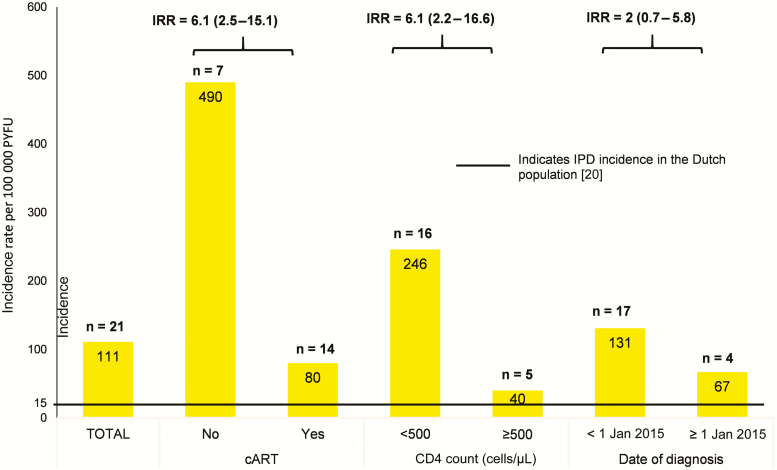
Incidence rates of IPD stratified to cART treatment and CD4 cell count are depicted in Figure 1. The incidence of IPD was the highest in patients without cART (490/100 000) or with a CD4 count <500 cells/μL (246/100 000) compared with patients with cART (80/100 000) or patients with a CD4 count >500 cells/μL (40/100 000) (Figure 1).
Figure 1.
Incidence (with 95% confidence intervals) of invasive pneumococcal disease in people living with human immunodeficiency virus, stratified by CD4 cell count and treatment status. Abbreviations: cART, combination antiretroviral therapy; IPD, invasive pneumococcal disease; IRR, incidence rate ratio; PYFU, patient-years of follow-up.
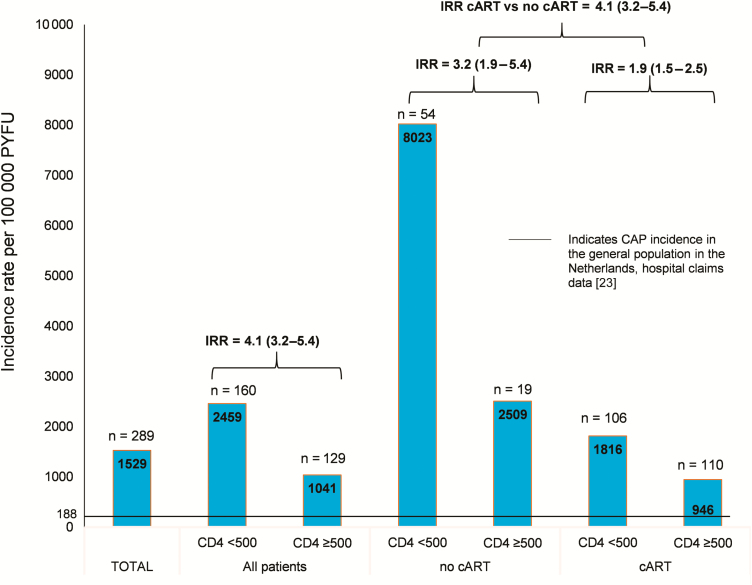
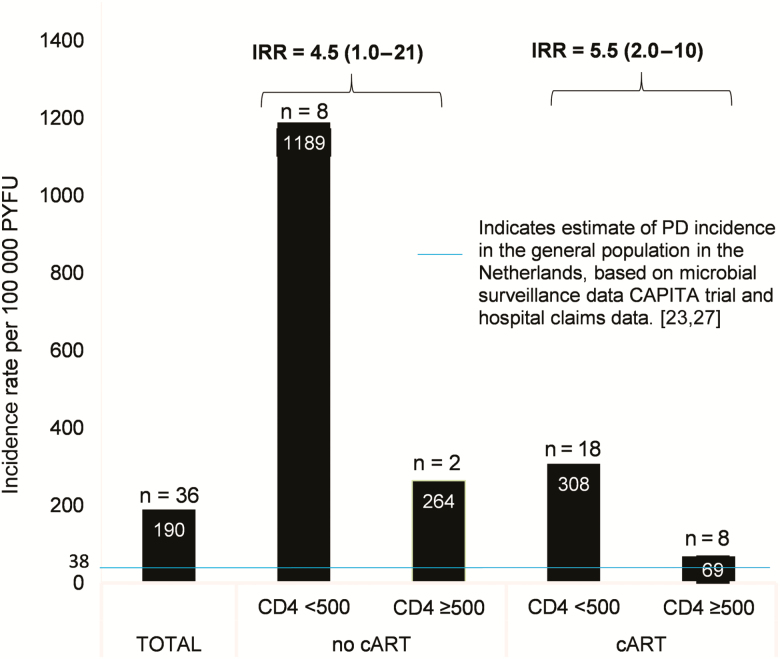
Similarly, for both CAP and PD (Figures 2 and 3), IRs were the highest in those without cART, combined with a CD4 count <500 cells/μL (8023/100 000 for CAP and 1189/100 000 for PD). Incidence rates were significantly lower in those receiving cART and with a CD4 count >500 cells/μL (946/100 000 for CAP and 69/100 000 for PD).
Figure 2.
Incidence (with 95% confidence intervals) of community-acquired pneumonia in people living with human immunodeficiency virus, stratified by CD4 count (cells/μL) and treatment status. Abbreviations: CAP, community-acquired pneumonia; cART, combination antiretroviral therapy, IRR, incidence rate ratio; PYFU, patient-years of follow-up.
Figure 3.
Incidence of pneumococcal disease (with 95% confidence intervals) in people living with human immunodeficiency virus, stratified by CD4 count (cells/μL) and treatment status. Abbreviations: CAPITA, Community-acquired Pneumonia Immunization Trial in Adults; cART, combination antiretroviral therapy; IRR, incidence rate ratio; PD, pneumococcal disease; PYFU, patient-years of follow-up.
Microbiology Data
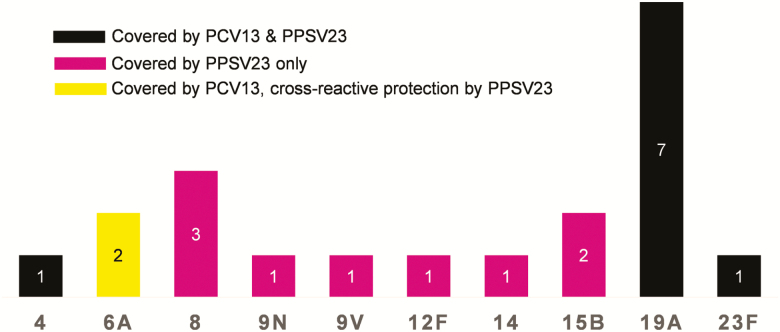
Streptococcus pneumoniae was the most commonly identified pathogen of CAP (30%). An overview of cultured pathogens is provided in Table 2. The serotype distribution of the pneumococcal isolates causing IPD is depicted in Figure 4. Data on microbial resistance of S. pneumoniae were available in 30 cases. In 2 (6.7%) cases, S. pneumoniae was penicillin-resistant, of which 1 strain was also resistant to amoxicillin and clavulanic acid, trimethoprim-sulfamethoxazole, and tetracyclines.
Table 2.
Proven Pathogens of Pneumonia
| Pathogens of Pneumoniaa | No. (%) (n = 119) |
|---|---|
| Streptococcus pneumoniae | 36 (30) |
| Haemophilus influenzae | 23 (19) |
| Pneumocystis jirovecii | 16 (14) |
| Virusesb | 13 (11) |
| Staphylococcus aureus | 6 (5.0) |
| Mycobacteriaceaec | 5 (4.0) |
| Klebsiella pneumoniae | 4 (3.0) |
| Pseudomonas aeruginosa | 4 (3.0) |
| Aspergillus | 3 (3.0) |
| Otherd | 9 (8.0) |
aA total of 119 pathogens were cultured in 104 patients.
bCytomegalovirus (n = 1); respiratory syncytial virus (n = 2); influenza A and B viruses (n = 9); parainfluenza virus (n = 4).
c Mycobacterium tuberculosis (n = 2); Mycobacterium avium (n = 2); Mycobacterium kansasii (n = 1).
d Legionella pneumophila (n = 1); Enterobacteriaceae (n = 1); Moraxella catarrhalis (n = 2); Entamoeba histolytica (n = 1); Cryptococcus (n = 1); Acinetobacter (n = 1); Escherichia coli (n = 1); Streptococcus mitis (n = 1).
Figure 4.
Number of cases per capsular serotype causing invasive pneumococcal disease. Abbreviations: PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
Case-control Study
Baseline characteristics of CAP cases and controls can be found in Table 3. Median age was higher in cases, whereas CD4 counts were significantly lower in cases. Significant risk factors for CAP in multivariable analysis were age >60 years, CD4 counts 200–500 cells/μL and <200 cells/μL, COPD, smoking, and drug use. Pneumococcal vaccination coverage was very low in both cases (7.4%) and controls (3.7%), with no significant difference (Table 4).
Table 3.
Community-acquired Pneumonia With Cases on Combination Antiretroviral Therapy Versus Controls: Univariable Conditional Regression Analysisa
| Characteristic | CAP Cases (n = 188)a | Controls (n = 188) |
P Value, χ 2 Test |
Crude OR (95% CI) |
|---|---|---|---|---|
| no./No. (%) | no./No. (%) | |||
| Female sex | 46/188 (25) | 55/188 (29) | .295 | 0.80 (.52–1.24) |
| Median age, y (IQR) | 53 (45–61) | 49 (41–56) | <.01 | NA |
| Age group, y | <.01 | |||
| 18–40 | 26/188 (14) | 45/188 (24) | ref | |
| 41–60 | 112/188 (60) | 121/188 (64) | 1.79 (.97–3.30) | |
| ≥61 | 50/188 (27) | 22/188 (12) | 4.09 (1.94–8.60) | |
| Mode of HIV acquisition | <.01 | |||
| Sex (heterosexual) | 57/176 (32) | 78/179 (44) | ref | |
| Intravenous drug use | 17/176 (9.7) | 3/179 (1.7) | 2.01 (1.17–2.46) | |
| MSM | 96/176 (55) | 89/179 (50) | 1.23 (.89–1.71) | |
| Mother to child | 4/176 (2.3) | 2/179 (1/1) | 1.58 (.57–4.35) | |
| Blood transfusion | 2/176 (1.1) | 7/179 (3.9) | 0.53 (.13–2.16) | |
| Ethnic group | .063 | |||
| White European | 129/184 (70) | 118/188 (63) | ref | |
| Asian | 8/184 (4.3) | 6/188 (3.2) | 1.09 (.54–2.24) | |
| Black African | 35/184 (19) | 57/188 (30) | 0.73 (.50–1.06) | |
| Hispanic | 12/184 (6.5) | 7/188 (3.7) | 1.21 (.67–2.19) | |
| Median CD4 count, cells/μL (IQR) | 530 (360–760) | 625 (452–839) | <.01 | NA |
| Median CD4 count category, cells/μL (IQR) | <.01 | |||
| <200 | 20/188 (11) | 4/188 (2.1) | ||
| 200–499 | 69/188 (37) | 57/188 (30) | ||
| ≥500 | 99/188 (53) | 128/188 (68) | ||
| Median nadir CD4 count, cells/μL (IQR) | (n = 186) 180 (88–283) | (n = 176) 170 (80–270) | .98 | NA |
| Detectable viral load time of episode | 32/188 (17) | 30/188 (16) | .78 | 1.11 (.59–2.10) |
| AIDS diagnosis prior to episode | 25/188 (13) | 6/188 (3.2) | <.01 | 4.80 (1.83–12.5) |
| No cART at time of episodeb | 0 | 16/188 (8.5) | NA | NA |
| Median time until cART initiation, mo (IQR)c | (n = 188) 22 (3–72) | (n = 171) 16 (1–47) | .06 | NA |
| Any comorbidity | 116/188 (62) | 83/118 (44) | <.01 | 2.07 (1.34–3.17) |
| Cardiovascular disease | 45/188 (24) | 37/188 (20) | .32 | 1.28 (.79–2.07) |
| COPD | 48/188 (26) | 8/188 (4.3) | <.01 | 7.67 (3.28–18.0) |
| Asthma | 7/188 (3.7) | 8/188 (4.3) | .79 | 0.88 (.32–2.41) |
| Diabetes | 13/188 (6.9) | 7/188 (3.7) | .16 | 2.00 (.77–5.33) |
| Asplenia | 3/188 (1.6) | 0 | .08 | 65 (.01–702 391) |
| Malignancy | 25/188 (13) | 24/188 (13) | .88 | 1.05 (.57–1.94) |
| Chronic kidney disease | 21/188 (11) | 6/188 (3.2) | <.01 | 4.00 (1.50–10.7) |
| Liver cirrhosis | 7/188 (3.7) | 3/188 (1.6) | .20 | 2.33 (.60–9.02) |
| Other immunodeficiencyc | 8/188 (4.3) | 0 | <.01 | 65.2 (.22–19 220) |
| Hepatitis C coinfectiond | 9/188 (4.8) | 20/188 (11) | .03 | |
| Hepatitis B coinfectione | 6/188 (3.2) | 12/188 (6.4) | .14 | 0.50 (0.19-1.33) |
| Smokingf | 100/188 (53) | 51/188 (27) | <.01 | 2.69 (1.76–4.12) |
| Alcoholg | 111/188 (59) | 94/188 (50) | .08 | 1.54 (.99–2.43) |
| Drug useh | 57/188 (30) | 24/188 (13) | <.01 | 2.83 (1.66–4.85) |
| Pneumococcal vaccinationi | 14/188 (7.4) | 7/188 (3.7) | .12 | 2.00 (.81–4.96) |
Boldface text indicates significant differences (P < .05).
Abbreviations: CAP, community-acquired pneumonia; cART, combination antiretroviral therapy; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; IQR, interquartile range; MSM, men who have sex with men; NA, not applicable; OR, odds ratio; ref, reference.
aOf the 188 included cases of CAP, 8 were bacteremic pneumococcal CAP (also invasive pneumococcal disease).
bOnly people living with HIV on cART were included in the case-control study. Controls were not selected based on cART use. Therefore, there are 16 cART-discordant case-control pairs.
cKidney transplant, immunoglobulin G subclass deficiency, use of immunosuppressive drugs for inflammatory diseases.
dChronic hepatitis C infection.
eActive hepatitis B infection or inactive carrier.
fOnly current smokers. We assumed that patients with missing data on smoking (eg, patient file did not mention smoking) did not smoke.
gWe assumed that patients with missing data on alcohol use (eg, alcohol used was not mentioned in the patient file by the HIV care provider) did not drink alcohol.
hAll use of drugs mentioned in the patient files, including recreational use (eg, cannabis, cocaine, opioids, amphetamines, ketamine, γ-hydroxybutyrate). If data on drug use were missing, we assumed that the patient did not use drugs.
iAdministration of any pneumococcal vaccine. Only 3 of 376 patients received both Prevenar13 and Pneumovax23 according to international guidelines.
Table 4.
Multivariable Conditional Logistic Regression Model With Cases and Controls Matched for Year of Human Immunodeficiency Virus Diagnosisa
| Covariatesb | Adjusted OR (95% CI) |
|---|---|
| Age group, y | |
| 18–40 | ref |
| 41–60 | 1.13 (.56–2.30) |
| ≥61 | 2.59 (1.09–6.18) |
| CD4 count, cells/μL | |
| <200 | 11.1 (2.85–42.9) |
| 200–499 | 1.89 (1.09–3.28) |
| ≥500 | ref |
| COPD | 3.07 (1.98–7.87) |
| Smokingc | 2.75 (1.56–4.85) |
| Drug used | 2.22 (1.12–4.37) |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
aThis analysis includes 16 combination antiretroviral therapy (cART)–discordant case-control pairs. We performed the same analysis excluding the 16 cART-discordant case-control pairs. This did not significantly change results (data not shown).
bCovariates in this model result from stepwise backward selection (P < .05).
cOnly current smokers. We assumed that patients with missing data on smoking (eg, patient file did not mention smoking) did not smoke.
dAll current use of drugs mentioned in the patient files, including recreational use (eg, cannabis, cocaine, opioids, amphetamines, ketamine, γ-hydroxybutyrate). If data on drug use were missing, we assumed that the patient did not use drugs.
DISCUSSION
We found that the incidence of IPD in PLHW was >7 times higher compared to the general Dutch population (14.9/100 000) [20] and >20 times higher than the incidence of IPD in healthy adults in the Netherlands (5/100 000) [14]. Compared to previous studies in high-income countries such as Denmark and the United Kingdom, we found lower incidence rates for IPD in PLHW [21, 22]. This may be due to the higher cART coverage and higher CD4 cell counts in our study. The incidence of CAP among PLWH in our study was >8 times higher compared to the general Dutch population (188/100 000) [23]. Incidence rates of CAP found in our study were comparable to previous studies in PLWH in high-income settings [24–26].
No surveillance data on PD (pneumococcal CAP + IPD) are available from the Netherlands. Based on the rate of pneumococcal infections reported in the Community-acquired Pneumonia Immunization Trial in Adults trial [27] and the overall incidence rate of CAP in the Netherlands [23], we estimate that the incidence of PD in PLWH is 5 times higher compared with the general population.
Among PLWH, we did not observe a decline in incidence of IPD, pneumococcal CAP, and CAP since 2015, when immediate initiation of cART was introduced [15]. However, this is not surprising, because even in current practice—when cART is immediately initiated in newly diagnosed PLWH, irrespective of the CD4 cell count—many patients still present late with significant immunodeficiency (CD4 count <350 cells/μL) or even with AIDS-defining opportunistic infections (45% in 2017 in the Netherlands). In addition, in 2017, 25% of all Dutch PLWH receiving cART were still immunocompromised, with a CD4 count <500 cells/μL [18].
Incidence rates of IPD, CAP and PD were consistently higher in the more severely immunocompromised (CD4 count <500 cells/μL) and untreated PLWH. Interestingly, also in optimally treated PLWH (virally suppressed and CD4 >500 cells/μL), incidence rates of IPD, CAP, and PD were still 4 times, 5 times, and 2 times higher, respectively, compared with the general population [20, 23, 27]. Our results are in agreement with a large study by Yin et al, who also report a residually increased incidence of IPD in optimally treated PLWH compared with the general population [22]. An explanation for this sustained risk of IPD and CAP in PLWH after CD4 recovery could be persisting humoral immune defects, including splenic dysfunction, after cART initiation, or additional risk factors [28]. Humoral immunity and splenic function are essential in protection against capsulated bacteria. Immediate initiation of cART after HIV diagnosis could potentially reduce the impact of these B-cell defects, although it is known that they occur early after HIV infection [21, 29, 30]. In patients receiving cART, a CD4 count <200 cells/μL, but also <500 cells/μL, was an independent risk factor for CAP. These findings are consistent with previous studies [1, 22, 31].
Other important risk factors for CAP in treated PLWH are similar to risk factors for CAP in the general population and include smoking, recreational drug use, and smoking [24, 28]. Cessation of smoking has been associated with a PD risk reduction in PLWH [25].
We found that all pneumococcal isolates were vaccine-covered serotypes (7 of 20 serotypes covered by PPSV23 only and 13 of 20 serotypes were covered by both 23-valent pneumococcal polysaccharide vaccine [PPSV23] and 13-valent pneumococcal conjugate vaccine [PCV13]) [8]. Our data, as well as the results of the study by Yin et al [21], indicate that in PLWH most IPD cases are still caused by vaccine serotypes. Several studies have shown that pneumococcal vaccination is safe and immunogenic in PLWH, particularly if PCV13 is followed by PPSV23, which is the current recommendation in most countries [7, 32–34]. Despite international recommendations, Dutch guidelines only recommend pneumococcal vaccination in PLWH with additional risk factors, such as asplenia and injecting drug use [35, 36]; this is reflected by a very low vaccination uptake in our study population (5.9%). However, even in the United States, vaccine uptake remains low in risk groups, including PLWH, despite longstanding recommendations for pneumococcal vaccination [5, 9, 10].
Due to the low pneumococcal vaccination rate in our study and the specific Dutch recommendations—vaccination of high-risk patients only, causing confounding by indication—our study could not provide reliable information on the effectiveness of pneumococcal vaccination.
Studies demonstrating the clinical benefit of pneumococcal vaccination in contemporary HIV cohorts are lacking. For conjugated vaccines, French et al reported a vaccine efficacy of 74% (95% confidence interval, 30%–90%) in preventing pneumococcal reinfection after 2 doses of 7-valent pneumococcal conjugate vaccine; however, this was in a low-income setting, with most patients untreated and at low CD4 cell counts [37]. Lamas et al showed that vaccination with PPSV23 was associated with a lower risk of pneumonia, but cocaine use and detectable viral load were much stronger predictors [31]. Altogether, there is an urgent need for data on the value of pneumococcal vaccination in PLWH. However, given the large numbers of patients that would be required to demonstrate clinical vaccine efficacy, such data may never be available. Therefore, due to the high burden of disease and good vaccine immunogenicity, vaccination with PCV13 followed by PPSV23 is recommended in most countries [8, 9] and is likely to be cost-effective [5]. Our study provides additional argumentation in favor of this approach.
This study highlights that even in a well-managed patient population in an academic HIV treatment center in a high-income country, PD and CAP remain important causes of morbidity. Our study has several limitations, of which the most important is that our IPD IRs were calculated on the basis of a relatively small number of cases. In addition, the rates of pneumococcal CAP and PD reported in our study are most likely an underestimation of the real disease burden, as the sensitivity of sputum culture is limited and urinary antigen tests were not performed during the study period. Last, databases that we used for collection of vaccine uptake figures did cover vaccinations that were administered at the general practitioner’s (GP) office. However, since Dutch guidelines do not recommend pneumococcal vaccination in PLWH, we do not expect that many patients received pneumococcal vaccines at the GP’s office [38].
CONCLUSIONS
The incidence of IPD and CAP among PLWH remains higher compared with the general population, even in those who are virally suppressed on cART and have high CD4 counts. Patients at additional increased risk are those with CD4 counts <500 cells/μL, smokers, recreational drug users, and patients with COPD. Although data on the clinical benefit of pneumococcal vaccination in PLWH are limited, our study demonstrates that the burden of pneumococcal disease remains high. With S. pneumoniae as the most commonly identified pathogen of CAP, and all serotyped pneumococcal isolates being covered by available pneumococcal vaccines, we provide additional argumentation against the poor current adherence to international recommendations for pneumococcal vaccination.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments.The authors thank Gertjan Wagenvoort, Stefan Vestjens, and other colleagues from the Antonius Hospital in Nieuwegein, the Netherlands, for their previous efforts in determining the human immunodeficiency virus status of all invasive pneumococcal disease (IPD) cases in the Netherlands between 2008 and 2016, and the Dutch HIV Monitoring Foundation for their efforts in registration of pneumonia episodes and providing us with data on patient-years of follow-up for people living with HIV who received care at the Amsterdam University Medical Centers, Location Acadamic Medical Center. The authors also thank their colleagues from the Netherlands Reference Laboratory for Bacterial Meningitis for their analysis of pneumococcal serotypes of IPD cases, as well as Remon Beukers for technical assistance with figure design.
Potential conflicts of interest.A. V. E. has received grants from Pfizer, other consultancy fees paid directly to the institution from GSK and participation in Science Advisory Boards for Pfizer. F. W. N. M. W. has received consultancy and speaker fees from ViiV Healthcare and Gilead Sciences. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Feikin DR, Feldman C, Schuchat A, Janoff EN. Global strategies to prevent bacterial pneumonia in adults with HIV disease. Lancet Infect Dis 2004; 4:445–55. [DOI] [PubMed] [Google Scholar]
- 2. van Aalst M, Lotsch F, Spijker R, et al. . Incidence of invasive pneumococcal disease in immunocompromised patients: a systematic review and meta-analysis. Travel Med Infect Dis 2018; 24:89–100. [DOI] [PubMed] [Google Scholar]
- 3. Grau I, Ardanuy C, Linares J, Podzamczer D, Schulze MH, Pallares R. Trends in mortality and antibiotic resistance among HIV-infected patients with invasive pneumococcal disease. HIV Med 2009; 10:488–95. [DOI] [PubMed] [Google Scholar]
- 4. Siemieniuk RA, Gregson DB, Gill MJ. The persisting burden of invasive pneumococcal disease in HIV patients: an observational cohort study. BMC Infect Dis 2011; 11:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine 2013; 31:6011–21. [DOI] [PubMed] [Google Scholar]
- 6. Krentz HB, Gill MJ. Cost of medical care for HIV-infected patients within a regional population from 1997 to 2006. HIV Med 2008; 9:721–30. [DOI] [PubMed] [Google Scholar]
- 7. Sadlier C, O’Dea S, Bennett K, Dunne J, Conlon N, Bergin C. Immunological efficacy of pneumococcal vaccine strategies in HIV-infected adults: a randomized clinical trial. Sci Rep 2016; 6:32076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lopez A, Mariette X, Bachelez H, et al. . Vaccination recommendations for the adult immunosuppressed patient: a systematic review and comprehensive field synopsis. J Autoimmun 2017; 80:10–27. [DOI] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816–9. [PubMed] [Google Scholar]
- 10. Williams WW, Lu PJ, O’Halloran A, et al. . Surveillance of vaccination coverage among adult populations—United States, 2015. MMWR Morb Mortal Wkly Rep 2017; 66:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valour F, Cotte L, Voirin N, et al. . Vaccination coverage against hepatitis A and B viruses, Streptococcus pneumoniae, seasonal flu, and A(H1N1)2009 pandemic influenza in HIV-infected patients. Vaccine 2014; 32:4558–64. [DOI] [PubMed] [Google Scholar]
- 12. Thornhill J, Sivaramakrishnan A, Orkin C. Pneumococcal vaccination in people living with HIV. Vaccine 2015; 33:3159–60. [DOI] [PubMed] [Google Scholar]
- 13. Heffernan RT, Barrett NL, Gallagher KM, et al. . Declining incidence of invasive Streptococcus pneumoniae infections among persons with AIDS in an era of highly active antiretroviral therapy, 1995–2000. J Infect Dis 2005; 191:2038–45. [DOI] [PubMed] [Google Scholar]
- 14. Wagenvoort GH, Knol MJ, de Melker HE, et al. . Risk and outcomes of invasive pneumococcal disease in adults with underlying conditions in the post-PCV7 era, the Netherlands. Vaccine 2016; 34:334–40. [DOI] [PubMed] [Google Scholar]
- 15. Lundgren JD, Babiker AG, Gordin F, et al. . Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV 2015. Available at: https://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/. Accessed March 23 2019.
- 17. Habib M, Porter BD, Satzke C. Capsular serotyping of Streptococcus pneumoniae using the Quellung reaction. J Vis Exp 2014; 84:e51208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stichting HIV Monitoring. HIV monitoring report 2017 Human immunodeficiency virus (HIV) infection in the Netherlands Available at: https://www.hiv-monitoring.nl/files/1115/1117/7706/HIV_Monitoring_Report_2017.pdf. Accessed 18 June 2018.
- 19. Boender TS, Smit C, Sighem AV, et al. . AIDS Therapy Evaluation in the Netherlands (ATHENA) national observational HIV cohort: cohort profile. BMJ Open 2018; 8: e022516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. European Centre for Disease Prevention and Control. Annual epidemiological report for invasive pneumococcal disease. Solna, Sweden: ECDC, 2016. [Google Scholar]
- 21. Harboe ZB, Larsen MV, Ladelund S, et al. . Incidence and risk factors for invasive pneumococcal disease in HIV-infected and non-HIV-infected individuals before and after the introduction of combination antiretroviral therapy: persistent high risk among HIV-infected injecting drug users. Clin Infect Dis 2014; 59:1168–76. [DOI] [PubMed] [Google Scholar]
- 22. Yin Z, Rice BD, Waight P, et al. . Invasive pneumococcal disease among HIV-positive individuals, 2000–2009. AIDS 2012; 26:87–94. [DOI] [PubMed] [Google Scholar]
- 23. Knol MJ, Sanders EAM, deMelker HE. Pneumokokkenziekte in Nederland: Achtergronddocument voor de Gezondheidsraad [in Dutch] Report from the National Institute for Public Health and the Environment 2017-0181. Available at: https://www.rivm.nl/bibliotheek/rapporten/2017-0181.pdf. Accessed 28 March 2019.
- 24. Sogaard OS, Reekie J, Ristola M, et al. . Severe bacterial non-AIDS infections in HIV-positive persons: incidence rates and risk factors. J Infect 2013; 66:439–46. [DOI] [PubMed] [Google Scholar]
- 25. Benard A, Mercie P, Alioum A, et al. . Bacterial pneumonia among HIV-infected patients: decreased risk after tobacco smoking cessation. ANRS CO3 Aquitaine Cohort, 2000–2007. PLoS One 2010; 5:e8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Curran A, Falco V, Crespo M, et al. . Bacterial pneumonia in HIV-infected patients: use of the pneumonia severity index and impact of current management on incidence, aetiology and outcome. HIV Med 2008; 9:609–15. [DOI] [PubMed] [Google Scholar]
- 27. Bonten MJ, Huijts SM, Bolkenbaas M, et al. . Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. New Engl J Med 2015; 372:1114–25. [DOI] [PubMed] [Google Scholar]
- 28. Curcio D, Cane A, Isturiz R. Redefining risk categories for pneumococcal disease in adults: critical analysis of the evidence. Int J Infect Dis 2015; 37:30–5. [DOI] [PubMed] [Google Scholar]
- 29. Moir S, Fauci AS. Insights into B cells and HIV-specific B-cell responses in HIV-infected individuals. Immunol Rev 2013; 254:207–24. [DOI] [PubMed] [Google Scholar]
- 30. Tsachouridou O, Skoura L, Zebekakis P, et al. . The controversial impact of B cells subsets on immune response to pneumococcal vaccine in HIV-1 patients. Int J Infect Dis 2015; 38:24–31. [DOI] [PubMed] [Google Scholar]
- 31. Lamas CC, Coelho LE, Grinsztejn BJ, Veloso VG. Community-acquired lower respiratory tract infections in HIV-infected patients on antiretroviral therapy: predictors in a contemporary cohort study. Infection 2017; 45:801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feikin DR, Elie CM, Goetz MB, et al. . Specificity of the antibody response to the pneumococcal polysaccharide and conjugate vaccines in human immunodeficiency virus–infected adults. Clin Diagn Lab Immunol 2004; 11:137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Farmaki PF, Chini MC, Mangafas NM, et al. . Immunogenicity and immunological memory induced by the 13-valent pneumococcal conjugate followed by the 23-valent polysaccharide vaccine in HIV-infected adults. J Infect Dis 2018; 218:26–34. [DOI] [PubMed] [Google Scholar]
- 34. Lu CL, Hung CC, Chuang YC, et al. . Serologic response to primary vaccination with 7-valent pneumococcal conjugate vaccine is better than with 23-valent pneumococcal polysaccharide vaccine in HIV-infected patients in the era of combination antiretroviral therapy. Hum Vaccin Immunother 2013; 9:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Landelijke Coördinatie Infectieziektebestrijding, Rijksinstituut voor Volksgezondheid en Mileu. Pneumokokkenziekte (invasief) richtlijn [in Dutch]. Bilthoven, the Netherlands: RIVM, 2007. [Google Scholar]
- 36. Nederlandse Vereniging van HIV Behandelaren. Vaccinatie tegen infecties met Streptococcus pneumoniae (pneumokokken) [in Dutch ]. Available at: https://lci.rivm.nl/richtlijnen/pneumokokkenziekte-invasief Accessed 10 September 2019. [Google Scholar]
- 37. French N, Gordon SB, Mwalukomo T, et al. . A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. New Engl J Med 2010; 362:812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De nationale HIV behandelrichtlijn. Guidelines Available at: https://richtlijnhiv.nvhb.nl/index.php/17.3._Vaccinatie_tegen_infecties_met_Streptococcus_pneumoniae_(Pneumokokken) Accessed 9 September 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.