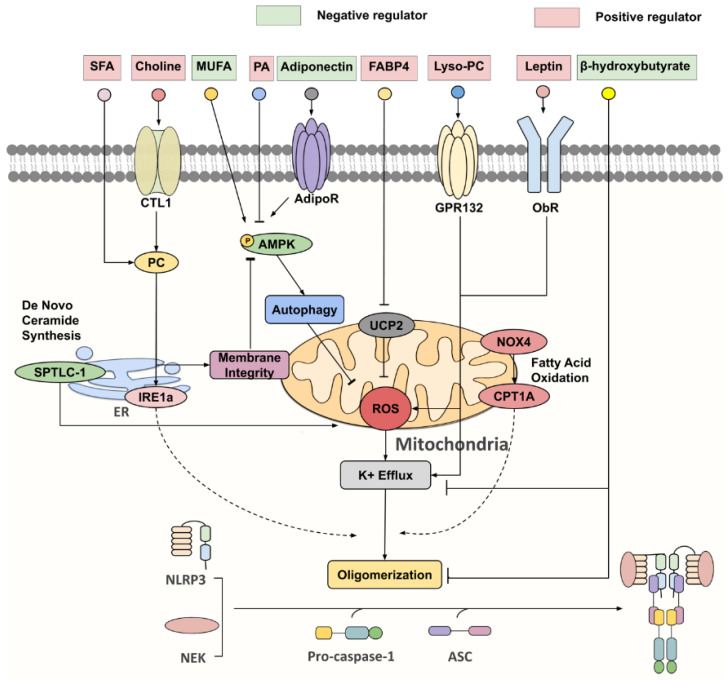
Figure 2.
Key negative and positive regulators for NLRP3 inflammasome. Under nutrient overload, SFAs [such as palmitic acid (PA)] and choline are extensively incorporated into phosphatidylcholine (PC), which activates inositol-requiring enzyme 1α (IRE1α), whose endonuclease activity promotes NLPR3 inflammasome activation via an undefined mechanism. Furthermore, PC synthesis through the choline pathway reciprocally regulates the AMP-activated protein kinase (AMPK)–autophagy–ROS signaling axis by maintaining mitochondrial membrane integrity. On the other hand, monounsaturated fatty acids (MUFA) and adiponectin were identified as initiators of AMPK-dependent autophagy, that attenuate ROS production and K+ efflux, thereby suppressing NLRP3 activation. FABP4, lyso-PC, leptin and serine palmitoyltransferase long chain base subunit 1 (SPTLC-1), a key enzyme involved in de novo ceramide synthesis, all partake in NLRP3 inflammasome activation via increasing ROS production. NADPH oxidase 4 (NOX4) enhances the protein expression of carnitine palmitoyl-transferase 1A (CPT1A), a rate-limiting fatty acid oxidation-related enzyme, which is responsible for heightening NLRP3 inflammasome response through a largely unknown pathway. β-hydroxybutyrate (BHB) was unveiled as a potent NLRP3 inflammasome inhibitor, targeting both K+ efflux and ASC oligomerization.