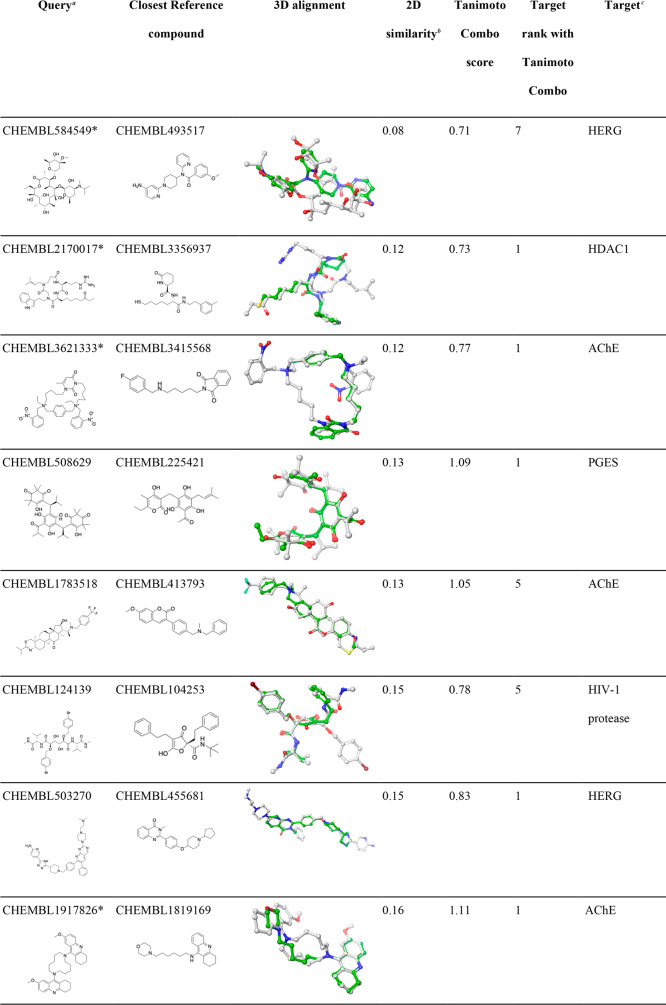
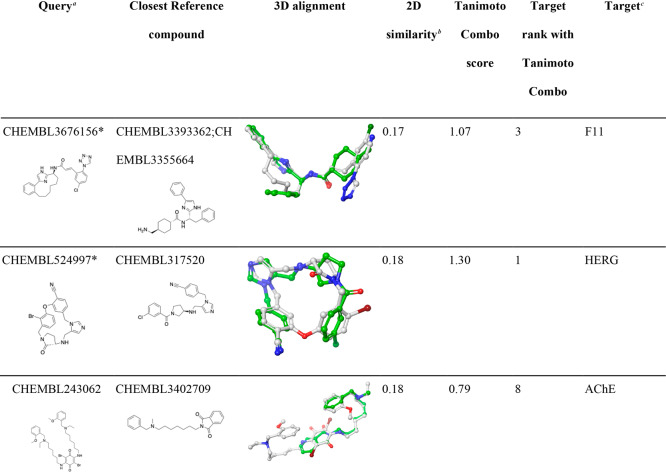
Table 6. Examples of CSMs for Which Their Targets Were Successfully Identified Despite Being Dissimilar from Any Reference Compound.
Queries marked with a “∗” are macrocyclic compounds.
2D molecular similarity between the CSM query and the closest ligand recorded in the knowledge base (measured as Tanimoto coefficient based on Morgan2 fingerprints).
HDAC1, histone deacetylase 1; AChE, acetylcholinesterase; PGES, prostaglandin E synthase; HIV-1 protease, human immunodeficiency virus type 1 protease; F11, coagulation factor XI.