Figure 3.
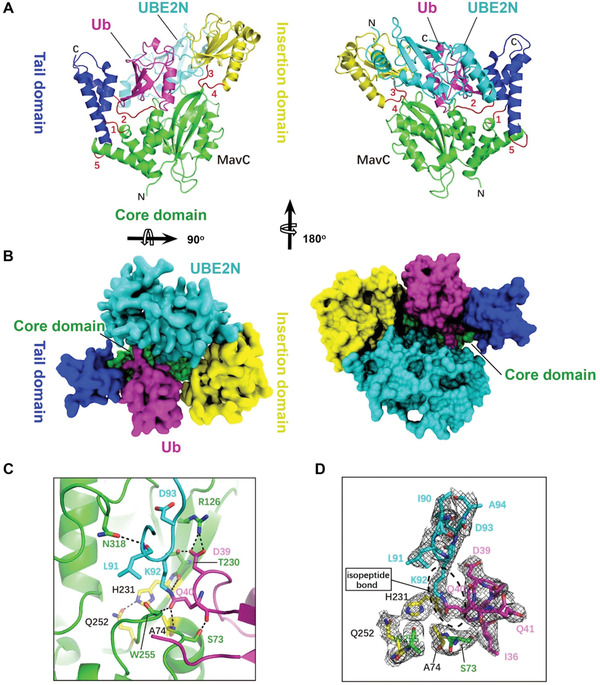
The overall structure of the MavC–UBE2N–Ub complex. A) Ribbon representation of the MavC–UBE2N–Ub ternary complex. In MavC, the Tail domain (helices α2, α3, and α14, blue) is linked to the Core domain (green) by three loops (loops 1, 2, and 5, red), whereas the Insertion domain (yellow) is linked to the Core domain by two loops (loops 3 and 4, red). Ub and UBE2N are shown in magenta and cyan, respectively. The view in right panel is generated by rotating the image in left panel by 180o around the indicated axis. B) Top view of the MavC–UBE2N–Ub ternary complex with surface representation. The color of the Tail domain, Core domain, Insertion domain, Ub, and UBE2N is shown same as (A). The view in right panel is generated by rotating the image in left panel by 180o around the indicated axis. C) MavC‐induced linkage between Lys92 of UBE2N (cyan) and Gln40 of Ub (magenta). The catalytic triad (yellow) of MavC (Cys74 was mutated to Ala in our structure) and other residues participating in the reaction are shown as sticks. Hydrogen bonds are shown as dashed lines. D) The 2Fo‐Fc map of key residues around active site contoured at 1.2 σ. The catalytic triad (Ala(Cys)74‐His231‐Gln252, yellow) and Ser73 of MavC, Ile90, Leu91, Lys92, Asp93, and Ala94 of UBE2N, and Ile36, Asp39, Gln40, and Gln41 of Ub are shown as sticks.
