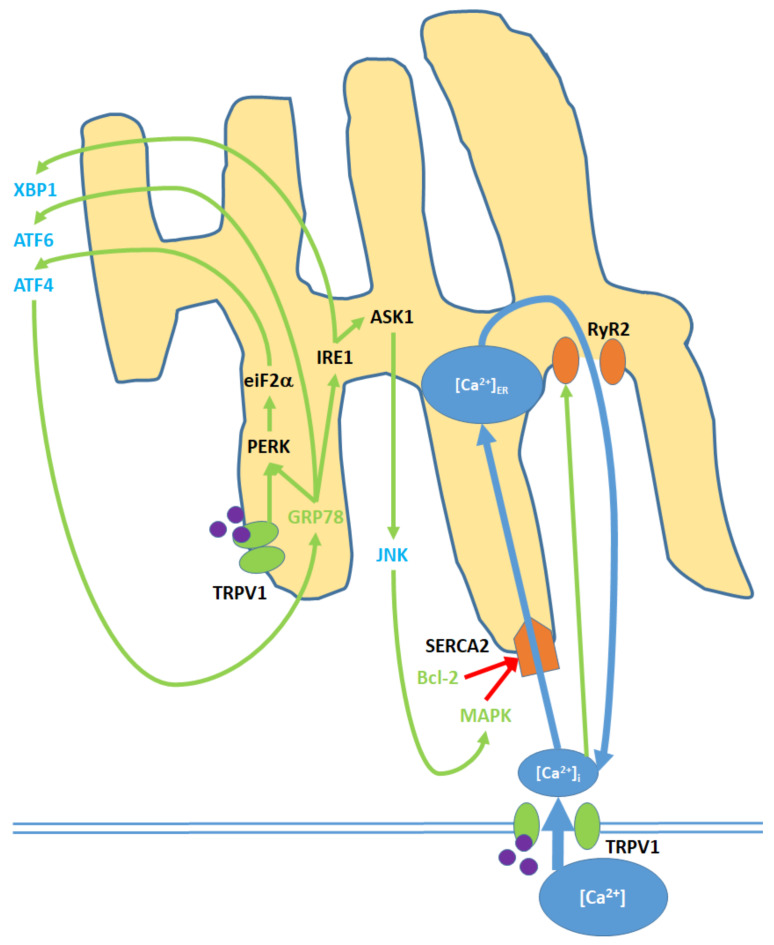
Figure 4.
Cell membrane and ER TRPV1 activation promote ER stress through the modulation of [Ca2+]ER, activation of various kinases, the upregulation of nuclear transcription factors, and the release of JNK into the cytosol. TRPV1 proteins localized to the ER membrane contribute only to protein signaling within the ER, while TRPV1 channels in the cell membrane promote both [Ca2+]i and protein signaling. Initial Ca2+ entry into the ER occurs through the SERCA2 pump, which is eventually blocked, causing net Ca2+ export via the RyR2 channels. GRP78 upregulation and Bcl-2 downregulation, as inputs, arise from nuclear activity. MAPK is both an input and output of ER stress, as it is upregulated via both mitochondrial activity and c Jun N-terminal kinases (JNK). ATF4, ATF6, and XBP1 are transcription factors that constitute the downstream nuclear targets of ER stress; ATF4, in particular, feeds back to the ER by upregulating GRP78.