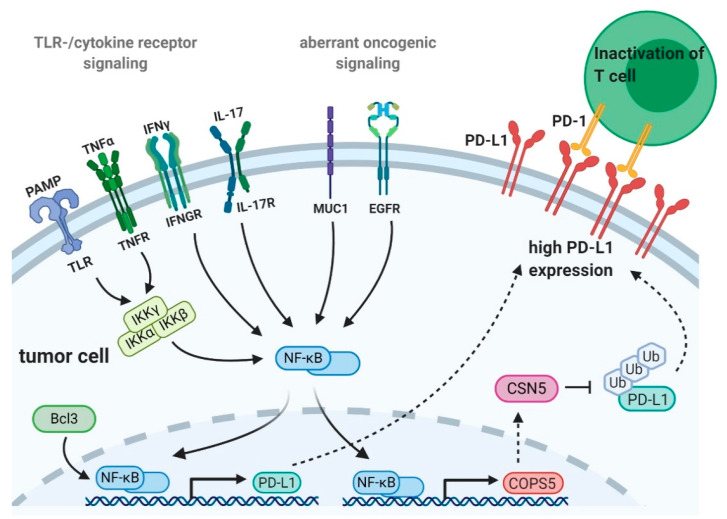
Figure 1.
Transcriptional and posttranslational regulation of programmed-death ligand 1 (PD-L1) by Nuclear factor-κB (NF-κB). Nuclear factor κB (NF-κB) is involved in transcriptional and posttranslational regulation of programmed-death ligand 1 (PD-L1) in immune and tumor cells. Toll-like receptor (TLR)- and cytokine receptor-signaling induce NF-κB activation and trigger its nuclear translocation enabling its binding to the PD-L1 promoter [34,55,67]. TLR- and tumor necrosis factor receptor (TNFR)-signaling activate the canonical NF-κB pathway by signaling via the IκB kinase (IKK) complex [7]. The exact mechanisms by which interferon γ (IFNγ) and interleukin-17 (IL-17) activate NF-κB are not completely understood. Aberrant expression of the oncogenes B cell lymphoma 3 (Bcl3) and mucin1 (MUC1) or epidermal growth factor receptor (EGFR) mutations are also described to induce NF-κB-mediated PD-L1 transcription [40,51,103]. NF-κB post-translationally regulates PD-L1 expression by inducing transcription of the COP9 signalosome complex subunit 5 (COPS5) gene encoding the fifth element of the COP9 signalosome (CSN5), which deubiquitinates and therefore stabilizes PD-L1 [38]. All of these mechanisms lead to high PD-L1 expression on tumor cells thereby contributing to tumor immune escape. Arrows indicate paths to NF-κB activation, dotted arrows indicate protein translation and translocation, T-bars indicate inhibition. Figure 1 was created with BioRender.com.