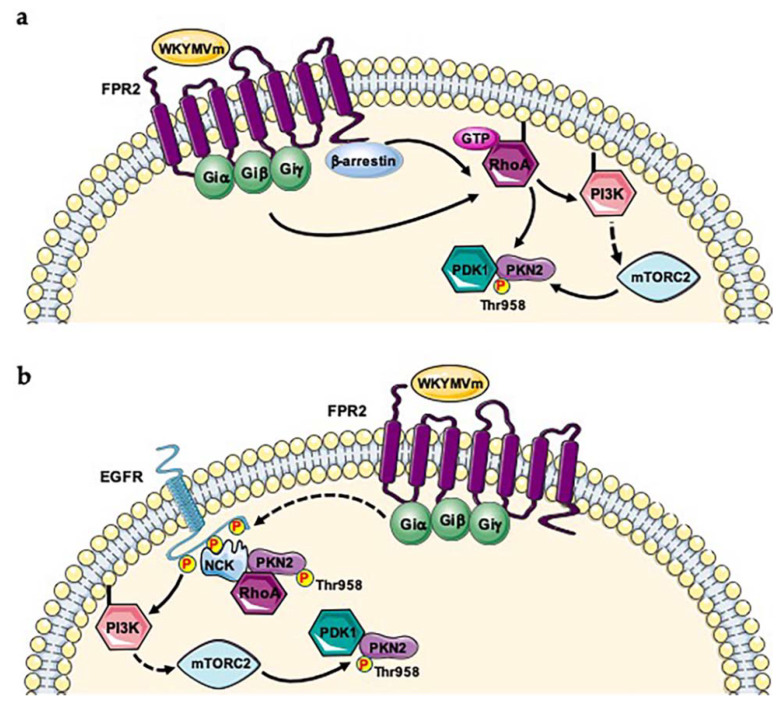
Figure 1.
FPR2 signaling induces Thr958 phosphorylation of PKN2. Two proposed mechanisms for PKN2 activation. (a) FPR2 stimulation induces Rho signaling allowing a conformational change in PKN2 and the phosphorylation in the activation loop by PDK1. FPR2 triggers Rho-dependent PI3K activation both in β-arrestin-dependent or -independent manner. PI3K activates mTORC2 which phosphorylates PKN2 at Thr958 residue. (b) Phospho-tyrosines of trans-phosphorylated EGFR provide docking sites for NCK binding and the SH3 domains of the adapter protein bind PKN2 and Rho. Rho induces a conformational change of PKN2 which is required for binding to PDK1 and the phosphorylation in the activation loop. EGFR-induced PI3K activity triggers PIP3 synthesis which is required for mTORC2 activation and Thr958 phosphorylation in the turn phosphate motif.