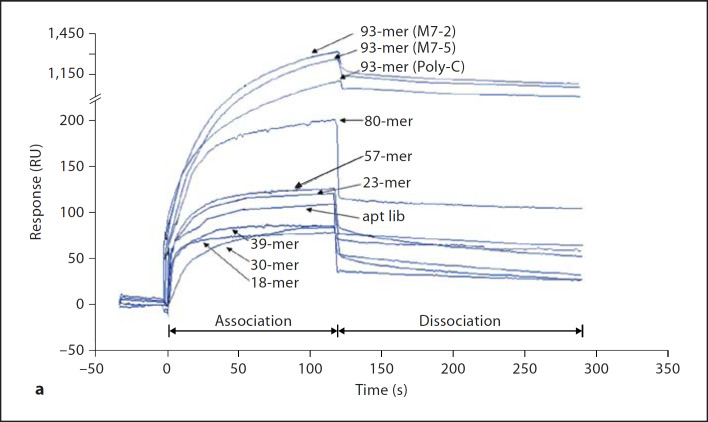
Fig. 6. a.
Sensorgrams depicting the real-time interaction between oligoaptamers of different lengths towards lipid A immobilized on a Biacore HPA chip. 60 µl aliquots of each of the nine oligoaptamers as well as aptamer library (‘apt lib’) at a series of different concentrations, ranging from 1, 0.4, 0.16 to 0.064 µM, were injected into the HPA chip at a flow rate of 30 µl/min with 100 mM Hepes, pH 9.0, containing 100 mM NaCl, 10 mM MgCl2 and 1.5% glycerol as the running buffer. During the dissociation phase, the running buffer was introduced for 120 s. For clarity, only sensorgrams of 0.4 µM of oligoaptamers are shown here. b The kinetic constant (KD values) of the oligoaptamers indicate the affinity of interaction between the oligoaptamers (of 18–93-mer) and lipid A, which was calculated using BIAevaluation software with a 1:1 Langmuir binding model.