Figure 4.
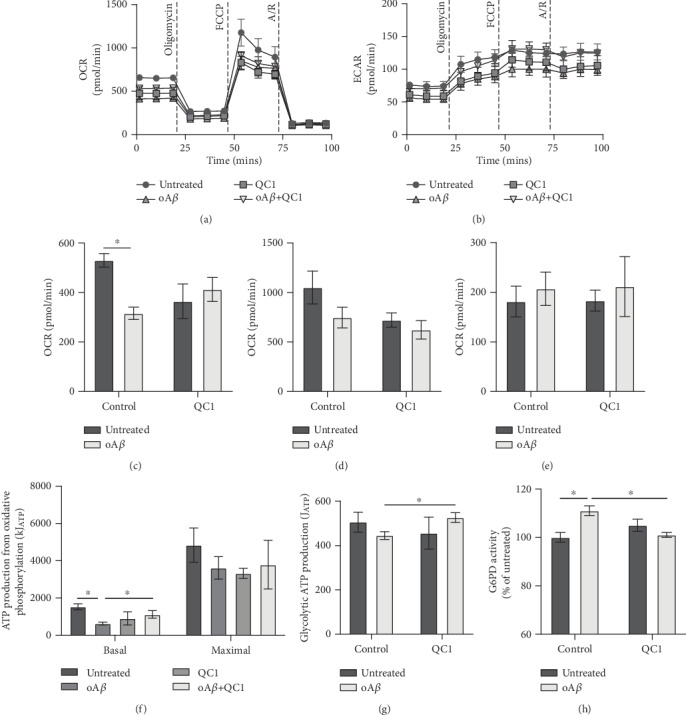
Treatment with oAβ suppresses mitochondrial respiration and promotes activity of the pentose phosphate pathway, effects reversed by subsequent activation of Fpr2/3. (a) Typical oxygen consumption rates of untreated BV2 cells and cells treated for 24 hrs with 100 nM oAβ with or without subsequent stimulation with QC1 (100 nM, 1 hr post-oAβ) and administration times for oligomycin (4 μM), FCCP (0.6 μM), and rotenone with antimycin A (both 1 μM) are indicated. (b) Typical extracellular acidification rates for untreated BV2 cells and cells treated for 24 hrs with 100 nM oAβ with or without subsequent stimulation with QC1 (100 nM, 1 hr post-oAβ) and administration times for oligomycin (4 μM), FCCP (0.6 μM), and rotenone with antimycin A (both 1 μM) are indicated. (c–e) Treatment with 100 nM oAβ for 24 hrs significantly suppressed basal metabolic rate (c), an effect that no longer reached statistical significance after QC1 treatment (100 nM, 1 hr post-oAβ). In contrast, neither oAβ nor QC1 treatment affected maximal respiration (d) or spare respiratory capacity (e). (f) Treatment with oAβ (100 nM, 24 hrs) significantly suppressed basal, but not maximal, ATP production due to mitochondrial oxidative phosphorylation, an effect reversed by subsequent treatment with QC1 (100 nM, 1 hr post-oAβ). (g) ATP generation from glycolysis was unaffected by either oAβ or QC1 treatment. (h) Activity of the rate-determining enzyme of the pentose phosphate pathway, glucose-6-phosphate dehydrogenase (G6PD), was significantly increased by treatment with 100 nM oAβ (24 hrs), an effect reversed by subsequent stimulation with 100 nM QC1 (1 hr post-oAβ). All data are mean ± SEM for 3-5 independent cultures, assayed in triplicate, ∗p < 0.05.
