Abstract
Severe acute respiratory syndrome coronavirus 2 is associated with higher concentrations of proinflammatory cytokines that lead to lung damage, respiratory failure, and resultant increased mortality. Immunomodulatory therapy has the potential to inhibit cytokines and quell the immune dysregulation. Controversial data found improved oxygenation after treatment with tocilizumab, an interleukin-6 inhibitor, sparking a wave of interest and resultant clinical trials evaluating immunomodulatory therapies. The purpose of this article is to assess potential proinflammatory targets and review the safety and efficacy of immunomodulatory therapies in managing patients with acute respiratory distress syndrome associated with coronavirus disease 2019.
Keywords: ARDS, COVID-19, cytokine storm, SARS-CoV-2, tocilizumab
Immunomodulatory therapies may potentially manage cytokine release syndrome and acute respiratory distress syndrome in patient with COVID-19, but efficacy and safety data are limited.
In December 2019, several cases of viral pneumonia were reported from Wuhan, China [1]. A novel coronavirus was identified, the genome of which was found to be 50% identical to Middle East respiratory syndrome (MERS-CoV) and 75%–80% identical to severe acute respiratory syndrome coronavirus (SARS-CoV), resulting in designation as SARS-CoV-2 [2]. Severe acute respiratory syndrome coronavirus has been noted to cause coronavirus disease 2019 (COVID-19) [2]. Thirty days after the initial report, 8600 confirmed cases of COVID-19 were reported across 30 provinces throughout China [3]. At day 90, there was a 90-fold increase in cases (775 000) worldwide with a 4.7% mortality rate [4].
As cases escalated, a common theme identified among patients was the presence of increased proinflammatory cytokines [5–7]. Similar to the cytokine production observed in patients with SARS-CoV who experienced acute lung injury (ALI) and resultant acute respiratory distress syndrome (ARDS), up to 30% of all patients with SARS-CoV-2 experience interstitial pneumonia that progresses to ARDS [8, 9]. A study from China reported a 62.5% mortality rate in critically ill patients with COVID-19 [10, 11]. Of all participants, 98% had recurrent fevers, 67% experienced ARDS, and 71% required mechanical ventilation (MV). High rates of organ failure occurred, including renal, hepatic, and cardiovascular. Other studies have reported elevated interleukin (IL)-6, C-reactive protein (CRP), and ferritin in patients with severe COVID-19 [9, 12]. These clinical findings are analogous to immune-mediated diseases such as secondary hemophagocytic lymphohistiocytosis (sHLH).
Secondary HLH is a hyperinflammatory syndrome commonly caused by viral infections [13]. Fatalities from sHLH are primarily due to severe endothelial dysfunction, disseminated intravascular coagulation (DIC), and multiorgan failure. Fifty percent of patients with sHLH experience persistent fevers, cytopenias, elevated ferritin, and respiratory failure [10]. Comparable proinflammatory markers, including IL-6, IL-18, interferon-γ, and ferritin, were observed in patients with sHLH and COVID-19, suggesting similar mechanism of inflammatory dysregulation between the 2 conditions [5–7, 13]. Presenting similarly to sHLH, cytokine release syndrome (CRS) is a hypothetical fatal systemic inflammatory response commonly associated with chimeric antigen T-cell (CAR-T) therapy, organ transplant, sepsis, and viral infections. Overlapping mechanisms and complications of these disorders have significantly influenced the management of COVID-19.
Controversial data from China reported treatment with tocilizumab, an IL-6 inhibitor, improved oxygenation, sparking a wave of interest and resultant clinical trials evaluating immunomodulatory therapies [14, 15]. The purpose of this article is to assess potential proinflammatory targets and review the safety and efficacy of immunomodulatory therapies in managing ARDS associated with COVID-19.
METHODS
A systematic literature search using PubMed, iSearch COVID-19 portfolio, World Health Organization (WHO) database of publications on COVID-19, Google Scholar, WHO International Clinical Trials Registry Platform (ICTRP), and clinicaltrials.gov was performed from December 21, 2019 to May 11, 2020. Key search terms included SARS-CoV-2 or COVID-19 and cytokine storm, ALI, ARDS, IL-1, IL-1RA, IL-6, JAK, AAK1, GAK, TH17 cells, VEGF, CD147, CD24Fc, and TNF-α. We included multiple spellings, truncated nomenclatures, and abbreviations in the search. Articles were screened by title and abstract for possible inclusion, and references within articles of interest were scanned to capture additional sources.
RESULTS AND DISCUSSION
Interleukin-6
Interleukin-6, a pleiotropic cytokine released by T-cells, endothelial cells, fibroblasts, macrophages, and monocytes during acute and chronic inflammatory disease, regulates the immune system through 2 pathways: classic and trans [16, 17]. In the classic pathway, IL-6 binds to membrane-bound IL-6 receptors (mIL-6R) on hepatocytes leading to the induction of the hepatic acute phase response and the release of CRP, hepcidin, a regulator of iron metabolism, and fibrinogen. This pathway is also associated with anti-inflammatory properties, such as intestinal epithelial cell proliferation and inhibition of epithelial cell apoptosis. In the trans pathway, IL-6 binds to soluble IL-6 receptor (sIL-6R) to form hyper-IL-6, a vastly more potent activator of gp130 receptors found on all nucleated mammalian cells. This binding activates the signal transducer and activator of transcription (STAT) 3, a transcription factor associated with cellular transformation, proliferation, and angiogenesis [18]. This leads to widespread hematopoiesis resulting in recruitment of mononuclear cells, inhibition of T-cell apoptosis, and inhibition of regulatory T-cell differentiation. In theory, hyper-IL-6 can activate all cells within the body, which explains its central role in cytokine storm.
Currently, there are 3 monoclonal antibodies capable of inhibiting IL-6 signaling: tocilizumab, sarilumab, and siltuximab (Table 1, Figure 1) [19–21]. Tocilizumab and sarilumab share an identical mechanism of action by inhibiting both the mIL-6R and sIL-6R, thereby preventing IL-6R activation and hyper-IL-6 formation. The mechanism of action for siltuximab differs in that it binds to IL-6 directly, resulting in the inhibition of IL-6R activation and hyper-IL-6 formation. However, all 3 lead to IL-6 signal inhibition.
Table 1.
Drug Information for FDA-Approved Therapies Under Consideration for Patients With COVID-19
| Generic Name (Brand Name) | Dose | Dose Adjustment | Contraindications/Adverse Effects (Listed in Alphabetic Order) | Potential Drug Interactions |
|---|---|---|---|---|
| IL-6 Inhibitors | ||||
| Tocilizumab (Actemra) [19] | Cytokine Release Syndrome • <30 kg–12 mg/kg IV • ≥30 kg–8 mg/kg IV (maximum 800 mg per IV infusion. Up to 3 additional doses 8 hours apart.) Giant cell arteritis/RA •162 mg SQ every 7 to 14 days Optimal dosage for COVID-19 unknown but doses of 4–8 mg/kg IV (maximum 800 mg/dose) that may be repeated in patients with suboptimal response are being studied in clinical trials |
No dose adjustments recommended in renal dysfunction Hepatic dysfunction: • ALT or AST >1−3× ULN: 4 mg/kg • ALT or AST >3−5× ULN: dose every other week • ALT or AST >5× UNL: discontinue |
• GI perforation • Hepatotoxicity • Hypersensitivity • Increased risk of infections including TB, IFI, opportunistic infections, and reactivation of HBV or VZV • Major adverse cardiovascular events • Neutropenia • Thrombocytopenia • Transaminitis |
• May increase or decrease metabolism of CYP450 substrates • Live vaccines should be avoided |
| Sarilumab (Kevzara) [20] | RA • 200 mg SQ every 2 weeks Optimal dosage for COVID-19 unknown but doses of 200 mg SQ and alternative IV dosing regimens are being studied in clinical trials |
No dose adjustments recommended in renal dysfunction Hepatic dysfunction: • ALT >3−5× ULN: interrupt therapy, may result once ALT <3× ULN • ALT >5× ULN: discontinue Neutropenia: • ANC 500–1000 cells/mm3: interrupt therapy, may resume once ANC >1000 cells/mm3 • ANC <500 cells/mm3: discontinue Thrombocytopenia: • Platelets 50 000–100 000 cells/mm3: interrupt therapy, may resume once platelets >100 000 cells/mm3 • Platelets <50 000 cells/mm3: discontinue |
• GI perforation • Hepatotoxicity • Hyperlipidemia • Hypersensitivity • Increased risk of infections including TB, IFI, opportunistic infections, and reactivation of HBV or VZV • Neutropenia • Thrombocytopenia • Transaminitis |
|
| Siltuximab (Sylvant) [21] | Castleman Disease • 11 mg/kg IV every 3 weeks Optimal dosage for COVID-19 unknown but doses of 11 mg/kg per day are being studied in clinical trials |
No dose adjustments recommended in renal or hepatic dysfunction Therapy should be delayed in patients with the following: • ANC <1000 cells/mm3 • Platelets <50 000 cells/mm3 • Hemoglobin ≥17 g/dL |
• Hepatotoxicity • Hypersensitivity • Increased hemoglobin • Increased risk of infections including TB, IFI, opportunistic infections, and reactivation of HBV or VZV • Neutropenia • Thrombocytopenia • Transaminitis |
|
| JAK Inhibitors | ||||
| Sunitinib (Sutent) [68] | GI stromal tumor, pancreatic neuroendocrine tumor, renal cell carcinoma • 37.5–50 mg PO every 24 hours, 4 weeks on, 2 weeks off Optimal dosage for COVID-19 unknown |
No dose adjustments recommended in renal or hepatic dysfunction Therapy should be modified or discontinued in patients with the following: • EF >20% but <50% below baseline without signs of HF • Signs or symptoms of HF • Severe hypertension • Dermatologic toxicities • Grade 3 or 4 hepatotoxicity • Thrombotic microangiopathy • Reversible posterior leukoencephalopathy syndrome • Nephrotic syndrome • Proteinuria (≥3 g/day) |
• Cardiotoxicity (including HR, cardiomyopathy, myocardial ischemia, and MI) • Embryo-fetal toxicity • Erythema multiforme • Hand-foot skin reaction • Hemorrhage • Hepatotoxicity • Hypertension • Necrotizing fasciitis • Neutropenia • Osteonecrosis of the jaw • Proteinuria/nephrotic syndrome • QTc prolongation • Stevens-Johnson syndrome (SJS) • Thrombotic microangiopathy • Toxic epidermal necrolysis (TEN) |
• Strong CYP3A4 inhibitors may increase sunitinib plasma concentration • Strong CYP3A4 inducers may decrease sunitinib plasma concentration |
| Erlotinib (Tarceva) [69] | Pancreatic Cancer • 100 mg PO every 24 hours Nonsmall Cell Lung Cancer • 150 mg PO every 24 hours Optimal dosage for COVID-19 unknown |
No dose adjustments recommended in renal or hepatic dysfunction Therapy should be delayed in patients with grade 3 or 4 renal toxicity or renal failure associated with hepatorenal syndrome or dehydration Concomitant administration with CYP3A4 inducers: increase by 50 mg Concomitant administration with CYP3A4 inhibitors: decrease by 50 mg | • Bullous and exfoliative skin disorders • Cardiovascular events (including cerebrovascular accidents, myocardial ischemia, and MI) • GI perforation • Hepatotoxicity • Interstitial lung disease • Microangiopathic hemolytic anemia • Ocular toxicity • Renal dysfunction and/or failure • Embryo-fetal toxicity |
• CYP3A4 and CYP1A2 inhibitor increase erlotinib plasma concentrations • CYP3A4 inducers decrease erlotinib plasma concentrations • Acid suppressive therapy |
| Ruxolitinib (Jakafi) [70] | Polycythemia vera • 50 mg PO every 24 hours Myelofibrosis • 20–50 mg PO every 24 hours Optimal dosage for COVID-19 unknown but doses of 10 mg PO every 12 hours × 14 days, then 5 mg PO every 12 hours × 2 days, then 5 mg PO every 24 hours × 1 day are being studied in clinical trials |
Therapy should be modified or discontinued in patients with the following: • Bleeding • CrCl <60 mL/min and platelets <150 000 cells/mm3 • Hepatic impairment (Child-Pugh class A, B, C) and platelets <150 000 cells/mm3 |
• Acute relapse of myelofibrosis symptoms • Anemia • Dizziness • Fatigue • Headache • Hyperlipidemia • Increased risk of infections including TB, IFI, opportunistic infections, and reactivation of HBV or VZV • Neutropenia • Nonmelanoma skin cancer • Thrombocytopenia |
• Strong CYP3A4 inhibitors, fluconazole |
| Fedratinib (Inrebic) [71] | Myelofibrosis • 400 mg PO every 24 hours Optimal dosage for COVID-19 unknown |
Renal dysfunction: • CrCl 15–29 mL/min: decrease dose to 200 mg every 24 hours Hepatic dysfunction: • Total bilirubin >3× ULN and any AST value: avoid use |
• Anemia • Encephalopathy including Wernicke’s • GI toxicity, • Hepatotoxicity, • Increased amylase and/or lipase • Neutropenia • Thrombocytopenia |
• Strong CYP3A inhibitors and inducers |
| Baricitinib (Olumiant) [72] | RA • 2 mg PO every 24 hours Optimal dosage for COVID-19 unknown but doses of 2–4 mg PO every 24 hours are being studied in clinical trials |
Renal dysfunction: • CrCl 30–60 mL/min: decrease dose to 1 mg every 24 hours • CrCl <30 mL/min: discontinue No dose adjustments recommended in hepatic dysfunction |
• Anemia • GI perforations • Hepatotoxicity • Hyperlipidemia • Increased risk of infections including TB, IFI, opportunistic infections, and reactivation of HBV or VZV • Lymphopenia • Malignancy • Neutropenia • Thrombosis |
• Live vaccines should be avoided • Organic Anion Transporter 3 (OAT3) inhibitors (eg, probenecid) |
| IL-1 Receptor Antagonists | ||||
| Anakinra (Kineret) [81] | Neonatal-Onset Multisystem Inflammatory Disease • 1–2 mg SQ every 24 hours (maximum dose 8 mg/kg per day) RA • 100 mg SQ every 24 hours Approved only for SQ administration in USA. Optimal dosage for COVID-19 unknown but multiple SQ and IV doses are being studied in clinical trials |
Renal dysfunction: • CrCl <30 mL/min or ESRD: administer every 48 hours No dose adjustments recommended in hepatic dysfunction |
• Cross-sensitivity to Escherichia coli-derived proteins • Hypersensitivity reactions including anaphylaxis • Increased risk of infections including TB, IFI, opportunistic infections, and reactivation of HBV or VZV • Injection site reactions • Malignancy • Neutropenia • Thrombocytopenia |
• Use with TNF-α inhibitors may increase risk of serious infections • Live vaccinations should be avoided |
| VEGF Inhibitors | ||||
| Bevacizumab (Avastin) [127] | Metastatic Colorectal Cancer • 5–7.5 mg/kg IV every 2 to 3 weeks Nonsmall Cell Lung Cancer • 15 mg/kg IV every 3 weeks Renal Cell Carcinoma • 10 mg/kg IV every 2 weeks Cervical Cancer • 15 mg/kg IV every 3 weeks Glioblastoma • 10 mg/kg IV every 2 weeks Ovarian, Fallopian Tube, or Peritoneal Cancer • 10–15 mg/kg IV every 2 to 3 weeks Do not administer for 28 days following major surgery Optimal dosage for COVID-19 unknown but doses of 7.5 mg/kg IV and 500 mg IV are being studied in clinical trials |
No dose adjustments recommended in renal or hepatic dysfunction Therapy should be modified or discontinued in patients with the following: • Nephrotic syndrome • Proteinuria (≥2 g/day) • Fistula formation involving any internal organ • GI perforation (any grade) • HF • Grade 3 or 4 hemorrhage • Hypertensive crisis • Hypertensive encephalopathy • Infusion reaction • Posterior reversible encephalopathy syndrome • Thromboembolic events • Wound healing complications |
• Delayed wound healing • Fetal toxicity • Fistula formation • GI perforations • Hemorrhage • HF • Hypertension • Ocular toxicity • Ovarian failure • Posterior reversible encephalopathy syndrome • Proteinuria/nephrotic syndrome • Thromboembolic events |
--- |
| TNF-α Inhibitors | ||||
| Adalimumab (Humira) [105] | RA, psoriatic arthritis, ankylosing spondylitis • 40 mg SQ every 2 weeks Plaque psoriasis, uveitis • 80 mg SQ on day 1, then 1 week later, 40 mg SQ every 2 weeks Crohn disease, ulcerative colitis, hidradenitis suppurativa • 160 mg SQ × 1, then 2 weeks later, 80 mg SQ × 1, then 2 weeks later, 40 mg SQ every 2 weeks Optimal dosage for COVID-19 unknown |
No dose adjustments recommended in renal or hepatic dysfunction | • Demyelinating disease • HF • Hypersensitivity • Increased risk of infections including TB, IFI, opportunistic infections, and reactivation of HBV or VZV • Malignancy • Neurologic reactions • Pancytopenia |
• Use with TNF-α inhibitors may increase risk of serious infections • Live vaccines should be avoided |
Abbreviations: ALT, alanine aminotransferase; ANC, absolute neutrophil count; AST, aspartate aminotransferase; COVID-19, coronavirus disease 2019; CrCl, creatinine clearance; CYP, cytochrome P450; EF, ejection fraction; ESRD, end-stage renal disease; FDA, US Food and Drug Administration; GI, gastrointestinal; HBV, hepatitis B virus; HF, heart failure; IFI, invasive fungal infections (including candidiasis and aspergillosis); IL, interleukin; IV, intravenous; JAK, Janus kinase; MI, myocardial infarction; OI, opportunistic infections (including pneumocystis); PO, by mouth; RA, rheumatoid arthritis; SQ, subcutaneous; TB, tuberculosis; TNF, tumor necrosis factor; ULN, upper limit of normal; VEGF, vascular endothelial growth factor; VZV, varicella zoster virus.
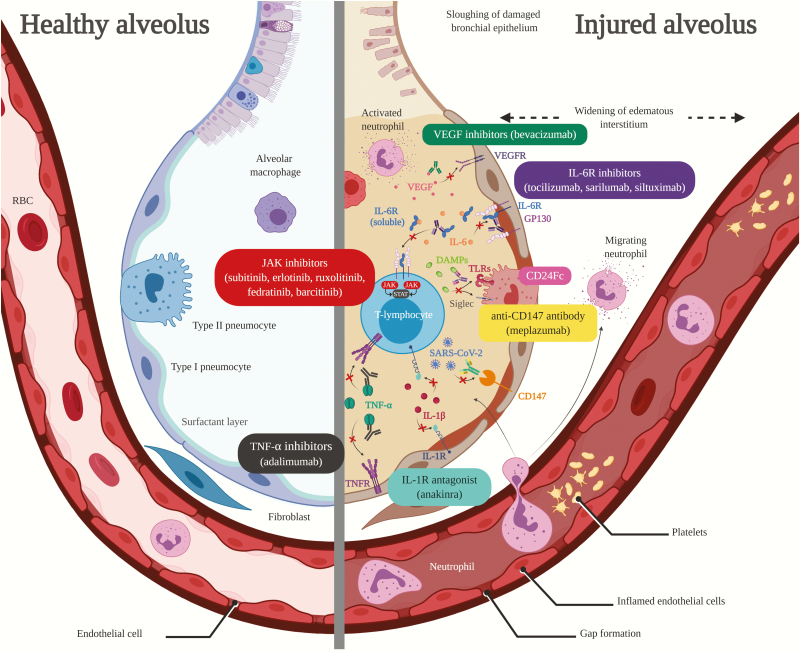
Figure 1.
Figure compares healthy alveolus (left) to injured alveolus (right) from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced proinflammatory host response and proposed targets of immunomodulatory therapy in coronavirus disease 2019 (COVID-19)-related acute lung injury/acute respiratory distress syndrome. Vascular endothelial growth factor inhibitor (VEGF) inhibitors (green text box) bind to and neutralize VEGF to inhibit pulmonary edema caused by VEGF overexpression. Interleukin-6 receptor (IL-6R) inhibitors (purple text box) inhibit both membrane-bound IL-6R and soluble IL-6R leading to inhibition of IL-6R activation and hyper-IL-6 formation, whereas siltuximab also binds directly to IL-6. Janus kinase (JAK) inhibitors (red text box) inhibit the activity JAK enzymes thereby interfering with the JAK/signal transducer and activator of transcription pathway responsible for inflammatory cytokine signaling. CD24Fc (pink text box) interacts with danger-associated molecular patterns (DAMPs) and sialic acid-binding Ig-like lectins (Siglecs) to inhibit nuclear factor-kappa B activation and release of inflammatory cytokines. Anti-CD147 antibodies (yellow text box) inhibit CD147, which may lead to decreased SARS-CoV-2 replication. Tumor necrosis factor (TNF)-α inhibitors (black text box) bind to TNF-α to prevent binding to TNF-α receptor (TNFR) sites and subsequent release of inflammatory cytokines. Interleukin-1R antagonists (blue text box) block activity of IL-1β by competitively inhibiting binding to IL-1R. RBC, red blood cell; TLR, Toll-like receptor. Created with BioRender.com.
These acute phase reactants may have a role in the manifestations of COVID-19, such as elevated ferritin and COVID-19-associated coagulopathy [22]. Elevated serum concentrations of IL-6 were found in patients with MERS-CoV, SARS-CoV, and SARS-CoV-2 [9, 23–25]. In those with SARS-CoV-2, postmortem biopsies showed significant elevations of IL-6 in the lungs. Interleukin-6 knockout mice had a profound defect mounting an antiviral T-cell response against influenza [26]. This was associated with enhanced infiltration of inflammatory monocytes into the lung, severe lung damage, extensive vascular leakage, and death. Based on these findings, blocking IL-6 seems counterintuitive for viral pneumonias.
During COVID-19 infection, serum IL-6 concentrations are increased above that of healthy controls, ranging from 13.3 pg/mL in nonsevere cases to 239 pg/mL in severe cases with ARDS [9, 14, 15, 27–29]. However, experimental administration of large doses of IL-6 to healthy volunteers did not cause ALI or ARDS [30]. In this experiment, subjects reached up to 4050 pg/mL of IL-6 without ALI, which is 16 times more than that observed during in severe COVID-19 [9, 12, 30]. It remains unclear whether increased IL-6 concentrations represent a marker and/or mediator of disease.
The initial study prompting the enthusiastic exploration of the effects of IL-6 inhibition in COVID-19 was a case series from China that included 21 severe or critically ill patients with COVID-19 treated with tocilizumab, plus standard of care (SOC), for cytokine storm (Table 2) [14, 15]. Oxygen requirements decreased in most patients, whereas body temperature normalized in all within 1 day after receipt of tocilizumab. C-reactive protein and lymphocytes returned to normal values. Chest computed tomography scans showed resorption of pulmonary lesions in almost all patients within 1 week. Posttreatment serum IL-6 concentrations remained elevated through day 5. As a result of these findings, tocilizumab was added to the Chinese treatment guidelines for COVID-19 [31]. Since then, multiple studies have been published describing clinical outcomes and effects on laboratory values of patients with COVID-19 after treatment with tocilizumab (Table 2) [28, 29, 32–35]. In addition, a press release from the open-label CORIMUNO-TOCI trial in France suggested significantly lower mortality and need for ventilator support in patients treated with tocilizumab with moderate to severe disease compared with placebo (data are unavailable) [36]. Analysis of preliminary results from the phase 2 portion of an ongoing phase 2/3 randomized study comparing low-dose (200 mg) and high-dose sarilumab (400 mg) to placebo in severe or critically ill patients with COVID-19 revealed no benefit in clinical outcomes or mortality when the severe and critical groups were compared with placebo (limited data available) [37]. Compared with placebo, negative trends were observed in the severe group, whereas a slight trend toward improved outcomes was observed in the critical group. As a result, the Independent Data Monitoring Committee amended the study going forward to only include critically ill patients to receive high-dose sarilumab or placebo. Interim analysis of the SISCO (siltuximab in serious COVID-19) study reported lower oxygen requirements (Table 2) [38]. Of 21 patients treated with siltuximab, one third were weaned off continuous positive airway pressure and noninvasive ventilation, 43% remained in stable condition, whereas 24% clinically deteriorated requiring MV. C-reactive protein and IL-6 serum concentrations were elevated in all patients with available baseline values. After treatment with siltuximab, CRP normalized by day 5, but posttreatment serum IL-6 concentrations were not reported. Although most studies reported CRP normalized after treatment, posttreatment IL-6 serum concentrations remained above the upper limit of normal. In patients treated with CAR-T therapy, tocilizumab inhibition of IL-6Rs led to higher serum IL-6 concentrations, despite normalizing inflammatory markers [39–41]. Greater reductions in serum IL-6 concentrations may be observed because siltuximab binds directly to IL-6, but the clinical benefits (eg, need for MV, intensive care unit length of stay, mortality, etc) are unknown. Data addressing these uncertainties will hopefully be provided in the multiple clinical trials assessing the efficacy of IL-6 inhibitors in patients with COVID-19 (Table 3) [42–57].
Table 2.
Available Evidence, Excluding Case Reports, for Use of Immunomodulatory Therapy in Patients With COVID-19
| Reference (Year) | Study Design (Location) | Patient Population | Intervention | Outcome(s) | Limitations/Critique |
|---|---|---|---|---|---|
| Tocilizumab | |||||
| Xu et al (2020) [14, 15] | Retrospective (Hefei, China) | Demographic data (n = 21) • Age, mean: 57 years • Male: 80% • Hypertension: 60% • Diabetes mellitus: 27% • Cerebrovascular disease: 5% • COPD: 5% • IL-6 concentration, mean (±SD): 153.44 (296.63) pg/mL • CRP concentration, mean (±SD): 75.06 (66.80) mg/L Severe (81%), defined as any of the following: • RR ≥30 bpm • SpO2 ≤93% on RA • PaO2/FiO2 ≤300 mmHg Critical (19%), defined as ICU admission plus any of the following: • MV • Shock • Organ failure |
• Tocilizumab 4–8 mg/kg (recommended dose 400 mg) IV once + SOCa | • Normalized body temperature within 24 hours: 100% • Decreased O2 requirements: 75% • Improvement in chest CT: 90.5% • No reports of adverse effects, pulmonary infections, clinical deterioration, or death • IL-6 concentration post-tocilizumab, mean (±SD) ◦ Day 1:129.18 (131.79) pg/mL ◦ Day 3: 300.98 (341.90) pg/mL ◦ Day 5: 274.90 (414.08) pg/mL • CRP concentration post-tocilizumab, mean (±SD) ◦ Day 1: 38.13 (54.21) mg/L ◦ Day 3: 10.61 (13.79) mg/L ◦ Day 5: 2.72 (3.60) mg/L |
• Small sample size • Nonrandomized • Limited description of methods and results • Time to tocilizumab administration unknown • 3 patients received a second dose of tocilizumab 400 mg IV within 12 hours due to sustained fever |
| Luo et al (2020) [29] | Retrospective (Wuhan, China) | Demographic data (n = 15) • Age, mean: 73 years • Male: 86% • Hypertension: 43% • Diabetes mellitus: 24% • Cerebrovascular disease: 20% • IL-6 concentration, mean (±SD): 111.1 (170.0) pg/mL • CRP concentration, mean (±SD): 131.81 (77.44) mg/L Moderately ill: 13% Seriously ill: 40% Critically ill: 47% |
• Tocilizumab IV on study day 0 | • Clinical improvement: 7% • Clinical stabilization: 60% • Disease aggravation: 13% • Death: 20% • IL-6 concentrations post-tocilizumab, mean (±SD) ◦ Day 1: 338.3 (383.4) pg/mL ◦ Day 3: 314.5 (317.3) pg/mL ◦ Day 5: 3196.8 (2191.4) pg/mL ◦ Day 7: 739.8 (1290.0) pg/mL • CRP concentration post-tocilizumab, mean (±SD) ◦ Day 1: 74.44 (30.24) mg/L ◦ Day 3: 21.61 (16.81) mg/L ◦ Day 5: 28.02 (22.18) mg/L ◦ Day 7: 14.15 (32.13) mg/L |
• Small sample size • Nonrandomized • Limited description of methods and results • Suboptimal reporting of safety profile • Initial tocilizumab doses included 80 mg (n = 2), 100 mg (n = 1), 240 mg (n = 1), 320 mg (n = 1), 400 mg (n = 3), 480 mg (n = 6), and 600 mg (n = 1) • 8 patients received tocilizumab in combination with methylprednisolone 20 mg to 80 mg IV every 12 to 24 hours • 5 patients received 2 or more doses of tocilizumab |
| Klopfenstein et al (2020) [32] | Retrospective, case-control comparing tocilizumab vs SOC (Trévenans, France) | Demographic data (tocilizumab group [n = 20] vs SOC group [n = 25]) • Age >70 years: 75% vs 44%, P = .036 • Charlson comorbidity index, mean (± SD): 5.3 (2.4) vs 3.4 (2.6), P = .014 • CRP concentration: 158 mg/L vs 105 mg/L, P = .017 |
• Tocilizumab • SOCb |
• Composite endpoint of death and/or ICU admission: 25% vs 72%, P = .002 • Death: 25% vs 48%, P = .066 • Receipt of invasive MV: 0% vs 32%, P = .006 |
• Small sample size • Nonrandomized • Scant demographic data available • Limited description of methods and results • Suboptimal reporting of safety profile • On average, tocilizumab administered 13 days after symptom onset, 7 days after admission, and after failure of SOC • Tocilizumab dosing regimen not defined |
| Toniati et al (2020) [28] | Case series (Brescia, Italy) | Demographic data (n = 100) • Age, median (IQR): 62 (57–71) years • Male: 88% • Hypertension: 46% • Diabetes mellitus: 17% • COPD: 9% • IL-6 concentration, median (IQR): 41 (10–102) pg/mL • CRP concentration, median (IQR): 113 (45–169) mg/L • BCRSS score, median (IQR): 3 (3–7) ◦ NIV: 57% ◦ MV: 43% |
• Tocilizumab 8 mg/kg (maximum dose 800 mg) IV × 2 doses, 12 hours apart + SOCc | 24–72 hours post-tocilizumab • Clinical and respiratory improvement: 58% • Clinical stabilization: 37% • Clinical worsening: 5% (of which 80% died) 10 days post-tocilizumab • Clinical and respiratory improvement or stabilization: 77% • Clinical worsening: 23% (of which 87% died) • IL-6 concentration, median (IQR): 1812 (375–2600) pg/mL • CRP concentration, median = 2 (IQR, 1–5) mg/L • BCRSS score, median = 2 (IQR, 1–4) |
• Nonrandomized • Limited description of methods and results • Used BCRSS, a 9-category, locally developed bedside respiratory severity scale (scores range from 0 [asymptomatic] to 8 [critically ill requiring MV and ICU management]) • Median (IQR) time from symptom onset to tocilizumab administration was 12 (9–14) days • Median (IQR) time from admission to tocilizumab administration was 5 (3–8) days • 13 patients received a third dose of tocilizumab 24 hours after the second based on clinical response • 3 adverse events were noted during the 10-day follow-up: 2 fatal cases of septic shock, 1 nonfatal case of gastrointestinal perforation |
| Roumier et al (2020) [33] | Retrospective, case-control comparing patients treated with tocilizumab vs patients not treated with tocilizumab (Suresnes, France) | Demographic data (tocilizumab group [n = 30] vs control group [n = 29]) • Age, mean (±SD): 58.8 (12.4) vs 71.2 (15.4) years, P = .001 • Male: 80% vs 79.3%, P = .947 • Hypertension: 20% vs 62.1%, P = .008 • Diabetes mellitus: 23.3% vs 34.5%, P = .343 • COPD: 13.3% vs 24.1%, P = .284 • ICU admission: 23.3% vs 34.5%, P = .079 • CRP concentration, mean (±SD): 189 (104.4) mg/L vs 167.4 (106.8) mg/L, P = .426 |
• Tocilizumab 8 mg/kg IV × 1 dose | Unadjusted analysis • Requirement of MV: 33.3% vs 55.2%, P = .089 • Death: 10% vs 31%, P = .04 Weighted analysis • Requirement of MV: 43.1% vs 64%, P = .025 • Death: 17.2% vs 18.7%, P = .837 |
• Nonpeer reviewed publication • Limited description of methods and results • Scant data on control group available • Suboptimal reporting of safety profile • Mean time from symptom onset to receipt of tocilizumab was 14.1 days • Patients could receive a second dose of tocilizumab if insufficient response, but number of patients who received second dose is unknown • 2 patients treated with tocilizumab also received HCQ 200 mg every 8 hours and azithromycin 250 mg every 12 hours on day 1, then every 6 hours thereafter |
| Sciascia et al (2020) [34] | Prospective open, single-arm multicenter (Torino, Italy) | Demographic data (n = 63) • Age, mean (±SD): 62.6 (12.5) years • Male: 88% • Hypertension: 38% • Diabetes mellitus: 9.5% • COPD: 4.7% Inclusion criteria: • SARS-CoV-2 PCR-confirmed COVID-19 infection • Pulmonary involvement defined as SpO2 ≤93% on RA or PaO2/FiO2 ≤300 mmHg • Hyperinflammation and hypercoagulable, defined as at least 3 of the following: ◦ CRP > 10× ULN ◦ Ferritin > 1000 ng/mL ◦ D-Dimer 10× ULN ◦ LDH 2× ULN |
• Tocilizumab 8 mg/kg IV (n = 34) • Tocilizumab 324 mg SQ (n = 29) |
• Mortality at 14 days: 11% o IV vs SQ: 12.9% vs 10.3%, OR = 1.16 (95% CI, 0.24–5.65), P = .858143 | • Limited description of methods and results • Suboptimal reporting of safety profile • Time to tocilizumab administration unknown • 52 patients received a second dose of tocilizumab within 24 hours of first dose • All patients were treated with concomitant antiviral therapy, of which 71.4% and 28.6% received LPV/r and DRV/c, respectively • No moderate or severe adverse events were observed |
| Colaneri et al (2020) [35] | Retrospective case-control comparing patients treated with tocilizumab vs patients not treated with tocilizumab (Pavia, Italy) | Demographic data (tocilizumab group [n = 21] vs control group [n = 91]) • Age, median (IQR): 62.33 (18.68) vs 63.74 (16.32) years • Male: 90% vs 69% • Hypertension: 38% vs 22% • Diabetes mellitus: 10% vs 9% • CRP concentration, median (IQR): 21.38 (13.40) mg/L vs 14.88 (14.41) mg/L |
• Tocilizumab + SOCd • SOCd |
• Mortality: OR = 0.78 (95% CI, 0.06–9.34) • ICU admission: OR = 0.11 (95% CI, 0.00–3.38) • CRP concentration post-tocilizumab, median (IQR): 0.63 (0.45) mg/L vs 6.07 (16.42) mg/L |
• Small sample size • Nonrandomized • Limited description of methods and results • Suboptimal reporting of safety profile • Tocilizumab dosing regimen not defined • Time to tocilizumab administration unknown • Severity of illness poorly described • No adverse effects were reported |
| Siltuximab | |||||
| Gritti et al (2020) [38]e | Retrospective (Bergamo, Italy) | Demographic data (n = 21) • Age, median: 64 years • Male: 86% • Hypertension: 43% • Diabetes mellitus: 24% • Cerebrovascular disease: 5% • IL-6 concentration, median (range): 139.5 (113–239) pg/mL • CRP concentration, median (range): 23.4 (9.5–43.1) mg/L ARDS requiring CPAP or NIV: 100% |
• Siltuximab 11 mg/kg per day IV within 2 days of CPAP or NIV | • Clinical condition improved: 33% • Clinical condition unchanged: 43% • Clinical condition worsened: 24% |
• Nonpeer reviewed publication • Small sample size • Nonrandomized • Limited description of methods and results • Suboptimal reporting of safety profile • Median initial siltuximab dose was 900 mg (range 700–1200 mg) • 5 patients received a second dose of siltuximab at physician’s discretion, as part of a compassionate-use program approved by the hospital ethics board |
| Anakinra | |||||
| Aouba et al (2020) [85] | Retrospective case series (France) | Demographic data (n = 9) • Age, median (range): 55 (46–84) years • Male: 89% • Hypertension: 33% • Diabetes mellitus: 11% • CRP concentration, median (range): 177 (83–282) mg/L Inclusion criteria: • Hospitalized, non-ICU • O2 flow ≤6 L/min • CRP ≥50 mg/L |
• Anakinra 100 mg SQ every 12 hours × 3 days, then 100 mg SQ every 24 hours × 7 days | CRP concentration post-anakinra, median (range) • Day 6: 19.5 (11–65) mg/L • Day 11: 4.5 (1–16) mg/L All patients were alive at last follow-up |
• Small sample size • Nonrandomized • Limited description of methods and results • Follow-up period unknown • Severity of illness poorly described • Suboptimal reporting of safety profile • Median (range) time from symptom onset to anakinra administration was 8 (4–12) days • Treatment was discontinued in one patient who developed acute respiratory failure after the first dose |
| Cavalli et al (2020) [27] | Retrospective cohort study comparing low-dose anakinra vs high-dose anakinra vs SOC (Milan, Italy) | Demographic data (low-dose anakinra [n = 167], high-dose [n = 29], SOC [n = 16]) • Age, median (IQR): 68 (51–73) vs 62 (55–71) vs 70 (64–78) years • Male: 71% vs 83% vs 88% • Hypertension: 43% vs 52% vs 50% • Diabetes mellitus: 29% vs 21% vs 19% • COPD: 14% vs 3% vs 13% • CRP concentration, median (IQR): 139 (109–172) vs 164 (105–227) vs 188 (130–246) mg/L Inclusion criteria: • NIV • Moderate-to-severe ARDS • Hyperinflammation defined as CRP ≥100 mg/L or ferritin ≥900 ng/mL • No evidence of bacterial infection • No concomitant use of other anti-inflammatory agents or corticosteroids |
• Low-dose anakinra (100 mg SQ every 12 hours) + SOCe • High-dose anakinra (5 mg/kg IV every 12 hours + SOCe • SOCe |
21 days post-anakinra (high-dose anakinra vs SOC) • Discharged from the hospital: 45% vs 44% • Survival: 90% vs 56% (HR = 0.20 [95% CI, 0.04–0.63], P = .009) • MV-free survival: 72% vs 50% (HR = 0.5 [95% CI, 0.16–1.30], P = .15) |
• Nonrandomized • Limited description of methods and results • Selection of treatment regimens unclear • Low-dose anakinra did not improve clinical status or CRP concentrations and was abandoned in favor of high-dose • Treatment continued until sustained clinical benefit, defined as 75% decrease in CRP, respiratory improvement for at least 2 days, or until death, bacteremia, adverse effects which equated to median (IQR) 9 (7–11) days with high-dose anakinra • Patients treated with high-dose who sustained clinical benefit were transitioned to anakinra 100 mg SQ every 12 hours × 3 days to prevent relapse • 7 patients discontinued high-dose anakinra due to adverse events after median (IQR) 9 (8–10) days of treatment • 3 patients discontinued high-dose anakinra due to increases in transaminases >3× ULN • 4 patients receiving high-dose anakinra developed Staphylococcus epidermidis bacteremia • 3 patients who did not improve after receiving high-dose anakinra were noted to have pulmonary emboli • All patients were treated with concomitant LPV/r and HCQ |
| Meplazumab | |||||
| Bian et al (2020) [128] | Prospective, single center, open-label case-control comparing patients treated with meplazumab vs patients not treated with meplazumab (Xi’an, China) | Demographic data (meplazumab group [n = 17] vs control group [n = 11]) • Age, median (IQR): 51 (49–67) vs 64 (43–67) years, P = .981 • Male: 64.7% vs 45.5%, P = .441 • Hypertension: 35.3% vs 27.3%, P = 1.0 • Diabetes mellitus: 17.6% vs 0%, P = .258 • COPD: 5.9% vs 0%, P = 1.0 Severity of illness • Common, defined as fever, respiratory symptoms, radiographic pneumonia: 23.5% vs 36.4% • Severe, defined as dyspnea, RR ≥30 bpm, SpO2 ≤93% on RA, or PaO2/FiO2 ≤300 mmHg: 35.3% vs 36.4% • Critical, defined as respiratory failure requiring MV, shock, multiorgan dysfunction/failure: 41.2% vs 27.3% |
• Meplazumab 10 mg IV × 1 dose on days 1, 2, and 5 + SOCd • SOCd |
Virologic Clearance Rate • Day 7: 76.5% vs 27.3% (P = .019) • Day 14: 94.1% vs 54.4% (P = .022) • Median: 3 days vs 13 days, P = .014 (HR = 0.37 [95% CI, 0.155–0.833]) Clinical improvement defined as normalized vital signs: • Day 7: 17.6% vs 0% • Day 14: 47.1% vs 27.3% • Day 21: 82.4% vs 54.5% • Day 28: 94.1% vs 81.8% |
• Nonpeer reviewed publication • Small sample size • Limited description of methods and results • All patients in both groups were treated with concomitant LPV/r and recombinant INFα-2b • 94.1% and 63.6% (P = .062) received concomitant systemic corticosteroids, respectively • 100% and 90.9% (P = .393) received concomitant antibiotics, respectively • Increased ALT and AST ≥2× ULN occurred in 2 patients receiving meplazumab |
Abbreviations: ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; BCRSS, Brescia COVID-19 respiratory severity scale; CI, confidence interval; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; CPAP, continuous positive airway pressure; CRP, C-reactive protein; CT, computerized tomography; DRV/c, darunavir/cobicistat; FiO2, percentage of inspired oxygen; HCQ, hydroxychloroquine; ICU, intensive care unit; IL, interleukin; INF, interferon; IQR, interquartile range; LPV/r, lopinavir/ritonavir; MV, mechanical ventilation; NIV, noninvasive ventilation; OR, odds ratio; PaO2, partial pressure of arterial oxygen; PCR, polymerase chain reaction; PMH, past medical history; RR, respiratory rate; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; SOC, standard of care; SpO2, peripheral oxygen saturation; SQ, subcutaneous; ULN, upper limit of normal.
aSOC included LPV/r 200/50 mg/tablet, 2 tablets PO every 12 hours × 10 days, INF-α 5 million units via inhalation every 12 hours, ribavirin 500 mg IV every 8 to 12 hours × 10 days, symptomatic therapy, ± methylprednisolone 1–2 mg/kg per day × 3–5 days in patients with rapid progression in respiratory dysfunction and/or imaging and excessive inflammatory response.
bSOC included HCQ or LPV/r ± systemic corticosteroids (doses and frequencies undefined).
cSOC included antiviral therapy [LPV/r 200/50 mg/tablet, 2 tablets PO every 12 hours or remdesivir 100 mg IV every 24 hours], antibacterial prophylaxis [azithromycin, ceftriaxone, or PTZ], HCQ 400 mg every 24 hours, and dexamethasone 20 mg every 24 hours. SOC included HCQ 200 mg PO every 12 hours + methylprednisolone 1 mg/kg (maximum dose of 80 mg) tapered × 10 days.
dSOC was undefined.
eSOC included HCQ 200 mg PO every 12 hours, LPV/r 200/50 mg/tablet, 2 tablets PO every 12 hours.
Table 3.
Ongoing or Planned Clinical Trials for Immunomodulators in Patients With COVID-19 (Data as of April 11, 2020)
| Clinical Trial Details | ||||||||
|---|---|---|---|---|---|---|---|---|
| Target | Proposed Mechanism vs SARS-CoV-2 or COVID-19 | Drug | Study Identifier (Location) | Study Type (Patient Population) | Intervention | Comparator(s) | Primary Outcome | Estimated Date of Completion |
| IL-6 | Diminish inflammatory dysregulation | Tocilizumab | NCT04317092 [42] (USA) | Prospective, observational, single arm (hospitalized, noncritically and critically ill) | • Tocilizumab 8 mg/kg IV × 2 doses, 12 hours apart | -- | Complete recovery (afebrile, normal SpO2) | March 31, 2021 |
| NCT04331795 [43] (USA) | Interventional, single arm (hospitalized, noncritically) | • Tocilizumab 200 mg IV × 1 dose (high dose) • Tocilizumab 80 mg IV × 1 dose (low dose) |
-- | Clinical and biochemical response | December 2020 | |||
| NCT04332094 [44] (Spain) | Randomized, multicenter, open-label, parallel assignment (hospitalized, noncritically and critically ill) | • Tocilizumab 162 mg SQ × 2 doses, 12 hours apart + HCQ 400 mg PO every 12 hours × 2 doses, then 200 mg every 12 hours × 6 days + azithromycin 500 mg PO every 24 hours × 3 days | • HCQ 400 mg PO every 12 hours × 2 doses, then 200 mg every 12 hours × 6 days + azithromycin 500 mg PO every 24 hours × 3 days | In-hospital mortality and need for MV in ICU | October 2020 | |||
| NCT04320615 [45] (USA) | Randomized, double-blind, multicenter, parallel assignment (hospitalized, noncritically and critically ill) | • Tocilizumab 8 mg/kg IV (up to 800 mg/dose) × 1 dose (an additional dose may be administered if symptoms worsen or no improvement) | • Placebo | Clinical status | September 30, 2021 | |||
| NCT04332913 [46] (Italy) | Prospective, observational cohort (hospitalized, noncritically and critically ill) | • Tocilizumaba | -- | Complete recovery (afebrile, normal SpO2) | March 31, 2021 | |||
| NCT04306705 [47] (China) | Retrospective cohort (3 study arms) (hospitalized, noncritically and critically ill) | • Tocilizumab 8 mg/kg IV × 1 dose | • CRRT • SOC |
Complete recovery (afebrile, normal SpO2) | June 20, 2020 | |||
| NCT04310228 [48] (China) | Randomized, multicenter, open-label, parallel assignment (3 study arms) (hospitalized, noncritically and critically ill) | • Tocilizumab 4–8 mg/kg (recommended dose 400 mg) IV × 1 dose (an additional dose may be administered if patient remains febrile within 24 hours after first dose) + favipiravir 1600 mg PO every 12 hours on day 1, then 600 mg PO every 12 hours on days 2 through 7 | • Tocilizumab 4–8 mg/kg (recommended dose 400 mg) IV × 1 dose (an additional dose may be administered if patient remains febrile within 24 hours after first dose) • Favipiravir 1600 mg PO every 12 hours on day 1, then 600 mg PO every 12 hours on days 2 through 7 |
Clinical cure (negative respiratory viral load) | May 2020 | |||
| NCT04333914 [49] (France) | Prospective, randomized, multicenter, parallel assignment (patients with advanced or metastatic hematological or solid tumor) | • Tocilizumab 400 mg IV × 1 dose | • Chloroquine analog (GNS651) 200 mg PO every 12 hours × 2 days, then 200 mg PO every 24 hours × 14 days • Nivolumab 0.3 mg/kg IV × 1 dose • SOC |
28-day survival | August 2020 | |||
| NCT04331808 [50] (France) | Randomized, multicenter, open-label, parallel assignment (3 study arms) (hospitalized, noncritically and critically ill) | • Tocilizumab 8 mg/kg IV × 1 dose (an additional dose may be administered if no improvement in O2 requirement within 48 hours) | • SOC | 14-day survival without need for MV | December 31, 2021 | |||
| NCT04315480 [51] (Italy) | Interventional, single arm, 2-stage (hospitalized, noncritically) | • Tocilizumab 8 mg/kg IV × 1 dose | -- | Arrest in deterioration of pulmonary function and improving in pulmonary function at 7 days | May 2020 | |||
| NCT04330638 [52] (Belgium) | Prospective, randomized, factorial design (6 study arms) (hospitalized, noncritically and critically ill) | • Tocilizumab 8 mg/kg IV (up to 800 mg/dose) × 1 dose | • Anakinra 100 mg SQ every 24 hours × 28 days or until hospital discharge • Siltuximab 11 mg/kg IV × 1 dose • Anakinra 100 mg SQ every 24 hours × 28 days or until hospital discharge + siltuximab 11 mg/kg IV × 1 dose • Anakinra 100 mg SQ every 24 hours × 28 days or until hospital discharge + tocilizumab 8 mg/kg IV (up to 800 mg/dose) × 1 dose • SOC |
Clinical improvement | December 2020 | |||
| Sarilumab | NCT04322773 [53] (Denmark) | Randomized, open label, sequential assignment (4 arms) (hospitalized, noncritically and critically ill) | • Sarilumab 200 mg SQ × 1 dose | • Tocilizumab 400 mg IV × 1 dose • Tocilizumab 162 mg SQ × 2 as a single dose • SOC |
Time to independence from supplementary oxygen therapy | June 1, 2020 | ||
| NCT04315298 [54] (USA) | Randomized, double-blind, placebo-controlled, parallel assignment (3 study arms) (hospitalized, critically ill) | • Sarilumab high dosea • Sarilumab low dosea |
• Placebo | Time to clinical improvement, percent change in CRP | April 1, 2021 | |||
| NCT04327388 [55] (USA) | Randomized, double-blind, placebo-controlled, parallel assignment (hospitalized, critically ill) | • Sarilumaba | • Placebo | Time to resolution of fever for at least 48 hours (phase II) Clinical severity (phase III) | June 2021 | |||
| NCT04324073 [56] (France) | Randomized, multicenter, open-label cohort (hospitalized, noncritically and critically ill) | • Sarilumab 400 mg IV × 1 dose | • SOC | 14-day survival without need for MV | December 31, 2020 | |||
| JAK, AAK1a, and GAKa (abaricitinib only) | Diminish inflammatory dysregulation, and inhibit receptor-mediated endocytosisa (abaricitinib only) | Baricitinib Ruxolitinib | NCT04321993 [57] (Canada) | Interventional, nonrandomized, open-label (hospitalized, noncritically and critically ill) | • Baricitinib 2 mg PO every 24 hours × 10 days | • LPV/r 200/50 mg/tablet, 2 tablets PO every 12 hours × 10 days • HCQ 200 mg PO every 12 hours × 10 days |
Clinical status | July 2021 |
| NCT04320277 [76] (Italy) | Interventional, nonrandomized, open-label, crossover assignment (hospitalized, noncritically and critically ill) | • Baricitinib 4 mg PO every 24 hours + LPV/r 200/50 mg/tablet, 1 tablet PO every 12 hours × 14 days | -- | ICU transfer | April 30, 2020 | |||
| ChiCTR2000029580 [77] (China) | Prospective, randomized, single blind, parallel assignment (hospitalized, noncritically and critically ill) | • Ruxolitiniba + mesenchymal stem cells | • SOC | Clinical improvement at 7 days and 1 month | December 31, 2020 | |||
| NCT04331665 [78] (Canada) | Interventional, open-label, single arm (hospitalized, noncritically and critically ill) | • Ruxolitinib 10 mg PO every 12 hours × 14 days, then 5 mg PO every 12 hours × 2 days, then 5 mg PO every 24 hours × 1 day | -- | Clinical deterioration | January 31, 2021 | |||
| IL-1RA | Diminish inflammatory dysregulation | Anakinra | NCT04324021 [86] (Italy) | Randomized, multicenter, open-label, parallel assignment (3 study arms) (hospitalized, noncritically) | • Anakinra 100 mg IV every 6 hours × 15 days | • Emapalumab 6 mg/kg IV on day 1, then 3 mg/kg IV on days 4, 7, 10, and 13 • SOC |
Treatment success, defined as proportion of patients not requiring MV or ECMO | September 2020 |
| VEGF | Decrease vascular permeability and pulmonary edema | Bevacizumab | NCT04305106 [93] (China) | Randomized, multicenter, parallel assignment (hospitalized, critically ill) | • Bevacizumab 7.5 mg/kg IV × 1 dose | • Placebo | Time to clinical improvement | July 31, 2020 |
| NCT04275414 [94] (China) | Interventional, single arm (hospitalized, noncritically and critically ill) | • Bevacizumab 500 mg IV × 1 dose + SOC | -- | PaO2/FiO2 ratio | May 2020 | |||
| CD147 | Block SARS-CoV-2 invasion of host cells | Meplazumabb | NCT04275245 [100] (China) | Interventional, single arm (hospitalized, noncritically and critically ill) | • Meplazumab 10 mg IV every 24 hours × 2 days | -- | Virologic clearance rate | December 31, 2020 |
| CD24Fc | Inhibit activation of NFkB and release of inflammatory cytokines | CD24Fc | NCT04317040 [102] (USA) | Randomized, multicenter, double-blind, placebo-controlled, parallel assignment (hospitalized, critically ill) | • CD24Fc 480 mg IV × 1 dose | • Placebo | Time to clinical improvement | May 2022 |
| TNF-α | Diminish inflammatory dysregulation | Adalimumab Adamumabc | ChiCTR2000030089 [106] (China) | Randomized, open-label, controlled trial (hospitalized, noncritically and critically ill) | • Adalimumaba + SOC | • SOC | Time to clinical improvement | August 31, 2020 |
| ChiCTR2000030580 [107] (China) | Prospective, randomized, single-center (hospitalized, critically ill) | • Adamumaba,c + tozumaba,d + SOC | • SOC | Chest CT imaging, virologic clearance rate, inflammatory markers | April 30, 2020 | |||
Abbreviations: AAK1, AP2-associated protein kinase 1; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CRRT, continuous renal replacement therapy; CT, computerized tomography; ECMO, extracorporeal membrane oxygenation; ECMO, extracorporeal membrane oxygenation; GAK, cycline G-associated kinase; HCQ, hydroxychloroquine; ICU, intensive care unit; IL, interleukin; JAK, Janus kinase; MV, mechanical ventilation; NFkB, nuclear factor-kappa B; RA, receptor antagonist; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOC, standard of care; SpO2, peripheral oxygen saturation; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor inhibitor.
aDosing regimen information unavailable.
bNot available in USA.
cTNF-α inhibitor unavailable in USA but considered a biosimilar to adalimumab.
dIL-6 inhibitor unavailable in USA but considered a biosimilar to tocilizumab [108].
Tocilizumab is effective in treating CRS secondary to CAR-T therapy, along with many other autoimmune diseases, including rheumatoid arthritis (RA) and Crohn’s disease, but it is associated with cardiovascular injury and increased risk of infections (Table 1). In 2 case reports, tocilizumab was successful in treating CRS secondary to CAR-T therapy and sHLH secondary to blinatumomab-associated CRS. After treatment, both patients were weaned off vasopressors and MV [58, 59]. Compared with tumor necrosis factor alpha (TNF-α) inhibitors in RA, tocilizumab had a higher risk for skin and soft tissue infections and diverticulitis [60]. Randomized control trials and real-world data suggest no clinically significant difference in cardiovascular risk between TNF-α inhibitors and tocilizumab in patients treated for RA [17]. In comparison to tocilizumab, sarilumab has a higher affinity for IL-6R and was more potent in inhibiting IL-6R activation and IL-6-induced cell proliferation [61]. The most common adverse effects of sarilumab were injection site reactions, neutropenia, and transaminitis [62]. Two studies comparing sarilumab against tocilizumab showed no clinically significant difference in incidence of treatment-emergent adverse events [63]. The incidence of neutropenia was numerically higher with sarilumab than with tocilizumab. However, neutropenia occurred more frequently with sarilumab compared with TNF-α inhibitors [64]. Due to the protective effects associated with the IL-6 classic signaling pathway, caution should be used in patients with intestinal perforation or diverticulitis. Bacterial, fungal, and viral infections should be ruled out before starting IL-6 inhibitors given the increased risk of infections and/or reactivation of latent infections.
Interleukin-6 inhibitors could potentially be effective treatment options for patients with COVID-19 with early signs of inflammatory dysregulation, but blocking one of many diverse cytokines may prove insufficient to quell the proinflammatory response. Furthermore, both short- and long-term safety data with IL-6 inhibitors in this patient population are unknown. Given the cardiovascular risk with both COVID-19 (reported in 29% of patients with COVID-19) and tocilizumab, patients should be monitored closely for cardiovascular injury [11]. Additional data are needed to establish the dosing, timing, and subsequent monitoring of IL-6 inhibitors before widespread use in patients with COVID-19.
Janus Kinase
Janus kinase (JAK) mediates the release of proinflammatory cytokines leading to increased inflammatory processes [65, 66]. Janus kinase inhibitors, sunitinib, erlotinib, ruxolitinib, fedratinib, and baricitinib, also have activity at AP2-associated kinase 1 (AAK1) and cyclin G-associated kinase (GAK) receptors, which are part of the numb-associated kinase family and play critical roles in regulating receptor-mediated endocytosis (Table 1) [67–72]. Sunitinib also acts as a vascular endothelial growth factor (VEGF) inhibitor, whereas erlotinib inhibits epidermal growth factor receptor inhibitor [73]. Fedratinib has a unique target different from the other JAK inhibitors, TH17 cells. These are a subtype of helper T-cells that produce IL-17, IL-21, IL-22, and granulocyte-macrophage colony-stimulating factor (GM-CSF). TH17 cells are activated through the JAK2-STAT pathway by IL-6 and IL-23. Fedratinib is more selective for JAK2, and by inhibiting it, the proinflammatory function of the TH17 cells may be restricted [74]. In addition, fedratinib decreased the expression of IL-17 by murine TH17 cells as well as IL-22 [74]. Other cytokines such as GM-CSF also use the JAK2 pathway so it is thought that fedratinib would inhibit these in a similar way.
The proposed mechanism behind JAK inhibitors is to inhibit cytokine signaling thereby downregulating the immune response related to SARS-CoV-2 (Figure 1) [65, 66]. In addition, SARS-CoV-2 is known to enter cells via endocytosis, and by inhibiting AAK1 and GAK, virus entry may become impaired. Ruxolitinib and fedratinib have lower affinity for AAK1 and GAK and would therefore require greater than currently tolerated therapeutic doses [66]. This suggests that ruxolitinib and fedratinib would be unlikely to inhibit receptor-mediated endocytosis but could still be used for JAK inhibition. Baricitinib has the potential to inhibit AAK1 and GAK at therapeutic doses, which may allow it to inhibit both cytokine signaling and receptor-mediated endocytosis of SARS-CoV-2 [66]. The doses of sunitinib and erlotinib needed to inhibit AAK1 and GAK are significantly higher than their normal therapeutic doses used for anticancer treatment, which would prove difficult for patients to tolerate and may lead to many unwanted adverse effects [65, 66].
Potential adverse reactions should be considered before use. Sunitinib, ruxolitinib, fedratinib, and baricitinib, are associated with neutropenia, lymphopenia, anemia, and thrombocytopenia [75]. Specifically, baricitinib should not be used in patients with an absolute lymphocyte count <500 cells/mm3 or absolute neutrophil count <1000 cells/mm3. Cytopenias have been seen in more severe cases of COVID-19, which could be exacerbated by most JAK inhibitors [75]. Reactivation of latent infections is associated with JAK inhibitors, and screening for bacterial, fungal, and viral infections should be completed before initiation.
Currently, baricitinib and ruxolitinib are the only JAK inhibitors being studied for use in COVID-19 patients (Table 3) [76–78]. There are 2 clinical trials underway comparing baricitinib with SOC, as well as other potential immunomodulatory therapies [76, 77]. In addition, there is an ongoing study exploring the use of ruxolitinib in combination with mesenchymal stem cells [78]. Understanding the potential adverse effects of these drugs is key, and weighing the risks versus benefits should be completed before consideration of use. Further evidence is needed to define the role of JAK inhibitors for patients with COVID-19 given the paucity of safety and efficacy data.
Interleukin-1
Interleukin-1 family comprises 11 cytokines and 10 receptors, which, compared with other cytokine families, are more associated with nonspecific, damaging inflammation [79]. Of these, IL-1α and IL-1β are responsible for direct and indirect host response. In addition, IL-1β is detectable in bronchoalveolar lavage fluid in patients with ARDS [80]. Conversely, the IL-1 receptor antagonist (IL-1RA) serves as a naturally occurring anti-inflammatory by inhibiting IL-1α and IL-1β [79]. Anakinra, a recombinant, nonglycosylated IL-1RA, has been evaluated as a method to counteract the cytokine storm observed in severe sepsis and septic shock (Table 1) [81]. Initial phase III trials failed to demonstrate decreased mortality [82, 83], but post hoc analysis found a significant 28-day survival benefit in patients with sepsis with multiorgan dysfunction syndrome and/or shock with concurrent DIC and hepatobiliary dysfunction [84].
Because IL-1β serum concentrations were significantly increased in patients with COVID-19 compared with healthy controls, it is thought that anakinra may block the activity of IL-1β in these patients (Figure 1) [12]. A small retrospective case series from France found lower CRP concentrations on day 6 after administration of a tapered regimen of subcutaneous anakinra over 3 days to 9 patients with moderate to severe COVID-19 (Table 2) [85]. The authors noted that CRP concentrations continued to trend down but remained elevated, and all patients were alive through day 11 of follow-up. A retrospective cohort study comparing low-dose anakinra and high-dose anakinra to SOC found higher survival rates but similar rates of MV-free survival at 21 days with high-dose anakinra (Table 2) [27]. Low-dose anakinra was not associated with improved clinical status or CRP concentrations and was abandoned. An open-label study comparing SOC with or without anakinra is expected to begin soon in Italy (Table 3) [86].
Although anakinra is well tolerated at standard doses, these lower doses did not prove beneficial. Limited data are available describing the tolerability and toxicity of high-dose anakinra. Additional data are needed to determine the dosing, timing, and subsequent monitoring of anakinra in patients with COVID-19.
Vascular Endothelial Growth Factor
Vascular endothelial growth factor is a potent vascular permeability inducer whose precise role in the lung remains unknown [87]. Higher concentrations of VEGF may promote increased vascular permeability and pleural effusions with resultant ALI [88]. In early ARDS, intrapulmonary VEGF concentrations are decreased whereas serum concentrations are increased [89], which is associated with poor prognosis [90]. As ARDS resolves, intrapulmonary and serum VEGF concentrations normalize [89]. Bevacizumab, a recombinant monoclonal antibody that binds to and neutralizes VEGF, was found to inhibit pulmonary edema in a VEGF overexpression model (Table 1, Figure 1) [91]. A study comparing bevacizumab to placebo in reducing incidence of ARDS in patients with severe sepsis was withdrawn due to lack of funding [92]. In comparison to healthy controls, VEGF serum concentrations were significantly increased in patients with COVID-19 [12]. Two clinical trials in China are currently recruiting participants to assess the efficacy of bevacizumab in patients with severe COVID-19 (Table 2) [93, 94]. The advantages of blocking VEGF in patients with COVID-19 must be weighed against the potential benefits of increased VEGF in respiratory distress [95, 96]. In addition, considerations as they pertain to the risk of serious adverse effects, including myocardial ischemia, cerebral thrombosis, and gastrointestinal perforation, are important in determining whether targeting VEGF is a viable option [97].
Emerging Options
CD147 is a transmembrane glycoprotein expressed in various tumors, inflamed tissues, and infected cells [98]. Previous data suggest that CD147 facilitates invasion of SARS-CoV into host cells, and therefore blocking CD147 may prevent viral invasion. Based on these findings, Wang et al [99] conducted an in vitro study to describe the interaction between SARS-CoV-2 and CD147 because of purported similarities between SARS-CoV and SARS-CoV-2. The authors noted that SARS-CoV-2 spike protein facilitated the invasion of host cells via CD147, and they observed decreases in viral replication when inhibiting CD147 with meplazumab, an anti-CD147 antibody (Figure 1). On the contrary, SARS-CoV N protein was found to bind to CD147 and not spike protein [98]. Nevertheless, a small prospective, single-center, open-label case-control study found faster virologic clearance in patients with COVID-19 treated with meplazumab (data not peer reviewed) (Table 2) [72]. Another clinical trial evaluating the effects of meplazumab on rate of viral clearance is currently recruiting in China (Table 3) [100]. More data are needed to determine the safety and efficacy of meplazumab in patients with COVID-19.
CD24Fc is a recombinant fusion protein consisting of the nonpolymorphic regions of CD24 attached to the Fc domain of human immunoglobulin (Ig)G1 [101]. Acting as an immunomodulator, CD24Fc may decrease inflammation associated with tissue injury by interacting with danger-associated molecular patterns and sialic acid-binding Ig-like lectins (Siglecs) thereby inhibiting nuclear factor-kappa B activation and release of inflammatory cytokines (Figure 1). Indeed, decreased CD8+ T cells, leukocyte infiltration, and IL-6 were observed after systemic administration to SIVmac239-infected macaques, but effects were more focused in the intestinal tract. The reasons for focused activity of CD24Fc in the gut remain unknown. A trial aimed at evaluating time to clinical improvement in patients with severe COVID-19 after administration of CD24Fc is anticipated to start soon in the United States (Table 3) [102].
TNF-α is a proinflammatory cytokine primarily produced by monocytes and macrophages. One of its many functions is to induce the production of other cytokines and promote inflammation [103]. Increased TNF-α concentrations in blood and tissue have been observed in some, but not all, patients with severe COVID-19 [104]. If inhibited, this may result in diminished inflammation (Figure 1). Currently, there are 2 clinical trials recruiting in China evaluating the use of TNF-α inhibitors (Table 1) [105] in COVID-19 patients (Table 2) [106, 107]. The first is a phase IV trial evaluating time to clinical improvement in patients who received either SOC or adalimumab in addition to SOC [106]. The second is a phase IV trial comparing SOC versus adamumab (unavailable in United States, but considered a biosimilar to adalimumab), tozumab (unavailable in United States, but considered a biosimilar to tocilizumab [108]), and SOC. This trial will be evaluating chest imaging, nucleic acid detection of virus, TNF-α, IL-6, and IL-10 in severe and critical COVID-19 patients [107].
Current Guideline Recommendations
As quickly as this situation is evolving, information available to guide treatment decisions is growing just as fast. Despite minimal evidence due to the short time frame there has been to develop it, there are varying recommendations pertaining to the use of immunomodulatory therapy in patients infected with SARS-CoV-2. Due to the evolving nature of this situation and limited number of guideline recommendations available, a general query web-search through Google was conducted on May 11, 2020 with the previously mentioned search terms. Institutional guidelines were screened by title for possible inclusion, and those focusing on special population of specific associated diagnosis were excluded. Of the varying immunomodulatory therapies discussed within this article, only the following are specifically named across a series of institutional, governmental, and societal guideline recommendations: tocilizumab, sarilumab, baricitinib, and ruxolitinib (Table 4) [31, 109–126].
Table 4.
Institutional, Governmental, and Societal Guideline Recommendations With Patient Specific Criteria for the Use of Immunomodulatory Therapy
| Immunotherapy discussed? (specific therapy named) | Guideline | Specific patient criteria |
|---|---|---|
| Yes (Tocilizumab) | SSC, SCCM ESICM [114] | None provided |
| Belgium [116] | Patients with critical disease • Persistent inflammation (e.g., elevated IL-6, CRP, D-Dimer, ferritin) and ARDS requiring mechanical ventilation without evidence of bacterial superinfection/sepsis |
|
| Brigham and Women’s Hospital [109] | Severe cases of COVID-19 with suspicion of cytokine activation syndrome • Use caution if secondary infection is clinically suspected |
|
| China [31] | Patients with extensive lung lesions and severe disease with elevated IL-6 concentration | |
| Italy [115] | Patients with critical disease and ARDS | |
| University of Washington [111] | Patients with severe disease with clinical deterioration despite other treatments • Persistent fever with temperature 38°C and one of the following ◦ Impending respiratory failure with increasing oxygen requirements or ARDS with a PaO2/FiO2 < 200 mm Hg ◦ New nonischemic cardiomyopathy requiring inotropic support and/or hypotension that requires on or more vasopressors ◦ Serum IL-6 concentration greater than 5x the upper limit |
|
| University of Mississippi Medical Center [122] | Consider in critically ill patients with suspected cytokine storm | |
| Amita Health [124] | Consider in critically ill patients with suspected cytokine storm | |
| Vanderbilt University [112] | Patients who clinically worsen despite other therapies | |
| Massachusetts General Hospital [120] | Patients with evidence of cytokine release syndrome | |
| Yes (Tocilizumab & Sarilumab) | University of Michigan [117] | Patients who cannot receive in a timely fashion or are not candidates for sarilumab trial Requirements: • Positive COVID-19 test • Abnormal chest imaging consistent with COVID-19 • Rapidly worsening gas exchange requiring >6 L/min O2 • Absence of systemic bacterial or fungal coinfection |
| Yale New Haven Health System [121] | Exclusion Criteria: • Anticipated immediate death (≤ 24 hours) regardless of critical care support • Cardiac: NYHA Class IV heart failure; Severe, inoperable multi-vessel coronary artery disease Cardiac arrest; Recurrent arrests in the current presentation, or unresponsive to defibrillation of pacing, or unwitnessed out-of-hospital cardiac arrest with poor prognosis • Hepatic: Cirrhosis with MELD-Na score ≥ 25 (in patient who are not transplant candidate), alcoholic hepatitis with MELD-NA ≥ 30, advanced liver cancer • Neurologic: Severe dementia leading to dependence in multiple ADLs; Rapidly progressive of end-stage neuromuscular disease • Oncologic: Advanced malignancy or high-grade primary brain tumors receiving only palliative treatment with estimated 3 or fewer months prognosis. • Pulmonary: Severe, chronic lung disease with baseline oxygen requirement of ≥ 60% FiO2; Primary pulmonary hypertension with NYHA Class III-IV heart failure (and patient refractor to/not a candidate for pulmonary vasodilators) • Trauma: Severe trauma; severe burns: age 60 years and 50% of total body surface area affected • Functional Status: dependent on all ADLs due to a progressive chronic comorbid condition |
|
| Yes (Tocilizumab, Baricitinib, Ruxolitinib) | Penn Medicine [118] | None provided |
| Yes (Tocilizumab) | Mount Sinai Health System [123] | Patients with severe disease with respiratory failure with no other end organ damage |
| None | NICE [110] | None provided |
| John Hopkins [119] | None provided | |
| American Thoracic Society [113] | None provided |
ADLs, activities of dialing living; ARDS, acute respiratory distress syndrome; CRP, C-reactive protein; ESICM, European Society of Intensive Care Medicine; FiO2, percentage of inspired oxygen; IL, interleukin; NICE, National Institute for Health and Care Excellence; NYHA, New York Heart Association; PaO2, partial pressure of arterial oxygen; SCCM, Society of Critical Care Medicine; SSC, Surviving Sepsis Campaign
Overall, there is varying guidance ranging from no mentions at all to supporting the use of immunotherapy under certain circumstances. The National Institute for Health and Care Excellence makes no mention of the use of immunomodulatory therapy among their available COVID-19 guidelines [110]. The American Thoracic Society makes no suggestion for or against the use of tocilizumab in hospitalized patients with COVID-19 [113]. Likewise, John Hopkins plans to provide recommendations specifically for the use of tocilizumab but has none at the time of writing [119].
Several recommendations exist supporting the use of immunomodulatory therapy in critically ill patients meeting specific criteria (Table 4) [31, 109, 111, 112, 115–117, 120–124]. Internationally, clinical guidance from China [31], Belgium [116], and Italy [115] recommends tocilizumab in specific patients. Stateside, the University of Michigan [117], University of Washington [111], Brigham and Women’s Hospital [109], Massachusetts General Hospital [120], Vanderbilt University [112], Yale New Haven Health System (YNHHS) [121], University of Mississippi Medical Center [122], Mount Sinai Health System [123], and Amita Health [124] provide specific recommendations for use of immunomodulatory therapy. Of the mentioned institutions, University of Michigan, YNHHS, and Mount Sinai Health System are involved with ongoing clinical trials with immunomodulatory therapy (eg, sarilumab) [117, 121, 123].
Recommendations against immunomodulatory therapy altogether are limited. The Society of Critical Care Medicine and European Society of Intensive Care Medicine jointly recommend against the use of tocilizumab due to insufficient evidence to issue a recommendation in critically ill adults [114]. Nebraska Medicine does not recommend the use of tocilizumab because of the unfavorable risk/benefit ratio [126]. Finally, Penn Medicine and University of Iowa Health Care discourage routine use of immunomodulatory therapy, highlighting lack of evidence [118, 125].
CONCLUSIONS
Severe acute respiratory syndrome coronavirus 2 leads to ALI and ARDS with increased mortality. Immunomodulatory therapies have the potential to inhibit cytokines, but the role of elevated cytokines with lung pathology is unclear. The overall lack of evidence and recommendations has forced practitioners to use their own judgment regarding use of immunomodulatory therapy. We are hopeful that as clinical trial data become available, their role in managing patients with COVID-19 will emerge. For now, available evidence suggests that these treatment options should be reserved for use in critically ill COVID-19 patients enrolled in clinical trials. Due to the potential adverse effects, risks and benefits must be weighed and proper screening must be completed before administration.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Zhu N, Zhang D, Wang W, et al. ; China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perlman S. Another decade, another coronavirus. N Engl J Med 2020; 382:760–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu Z. CROI Special Session on COVID-19: 15-minute live video update from China 2020. Available at: https://special.croi.capitalreach.com/. Accessed 6 April 2020.
- 4. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020; 20:533–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu XW, Wu XX, Jiang XG, et al. . Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 2020; 368:m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mahallawi WH, Khabour OF, Zhang Q, et al. . MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine 2018; 104:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wong CK, Lam CW, Wu AK, et al. . Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 2004; 136:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tisoncik JR, Korth MJ, Simmons CP, et al. . Into the eye of the cytokine storm. Microbiol Mol Biol Rev 2012; 76:16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mehta P, McAuley DF, Brown M, et al. ; HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang X, Yu Y, Xu J, et al. . Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Resp Med 2020; 8:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karakike E, Giamarellos-Bourboulis EJ. Macrophage activation-like syndrome: a distinct entity leading to early death in sepsis. Front Immunol 2019; 10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu X, Han M, Li T. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv [Preprint]. 2020:202003.00026. Available at: http://www.chinaxiv.org/abs/202003.00026. Accessed 6 April 2020. [DOI] [PMC free article] [PubMed]
- 15. Xu X, Han M, Li T, et al. . Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020; 117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rose-John S. The soluble interleukin 6 receptor: advanced therapeutic options in inflammation. Clin Pharmacol Ther 2017; 102:591–8. [DOI] [PubMed] [Google Scholar]
- 17. Zhang C, Wu Z, Li JW, et al. . Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents 2020; 55:105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aggarwal BB, Kunnumakkara AB, Harikumar KB, et al. . Signal transducer and activator of transcription-3, inflammation, and cancer. Ann N Y Acad Sci 2009; 1171:59–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Actemra (Tocilizumab) [prescribing information]. South San Francisco, CA: Genentech Inc; 2019. [Google Scholar]
- 20. Kevzara (Sarilumab) [prescribing information]. Bridgewater, NJ: Sanofi-Aventis; 2018. [Google Scholar]
- 21. Sylvant (Siltuximab) [prescribing information]. Hemel Hempstead, Hertfordshire, United Kingdom: EUSA Pharma (UK); 2019. [Google Scholar]
- 22. Tang N, Bai H, Chen X, et al. . Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020; 18:1094–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Castilletti C, Bordi L, Lalle E, et al. . Coordinate induction of IFN-alpha and -gamma by SARS-CoV also in the absence of virus replication. Virology 2005; 341:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shi SQ, Peng JP, Li YC, et al. . The expression of membrane protein augments the specific responses induced by SARS-CoV nucleocapsid DNA immunization. Mol Immunol 2006; 43:1791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tseng CT, Perrone LA, Zhu H, et al. . Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection. J Immunol 2005; 174:7977–85. [DOI] [PubMed] [Google Scholar]
- 26. Lauder SN, Jones E, Smart K, et al. . Interleukin-6 limits influenza-induced inflammation and protects against fatal lung pathology. Eur J Immunol 2013; 43:2613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cavalli G, De Luca G, Campochiaro C, et al. . Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatology 2020; 2:E325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Toniati P, Piva S, Cattalini M, et al. . Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev 2020; 19:102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luo P, Liu Y, Qiu L, et al. . Tocilizumab treatment in COVID-19: a single center experience. J Med Virol 2020. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsigos C, Papanicolaou DA, Kyrou I, et al. . Dose-dependent effects of recombinant human interleukin-6 on glucose regulation. J Clin Endocrinol Metab 1997; 82:4167–70. [DOI] [PubMed] [Google Scholar]
- 31. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment. 7th ed. China National Health Commission; 2020. Available at: http://kjfy.meetingchina.org/msite/news/show/cn/3337.html. Accessed 27 April 2020. [Google Scholar]
- 32. Klopfenstein T, Zayet S, Lohse A, et al. . Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients [published online ahead of print May 6, 2020]. Med Mal Infect 2020. doi: 10.1016/j.medmal.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roumier M, Paule R, Groh M, et al. . Interleukin-6 blockade for severe COVID-19. 2020. doi: 10.1101/2020.04.20.20061861. [DOI]
- 34. Sciascia S, Aprà F, Baffa A, et al. . Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol 2020; 38:529–32. [PubMed] [Google Scholar]
- 35. Colaneri M, Bogliolo L, Valsecchi P, et al. . Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms 2020; 8:E695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Assistance Publique - Hôpitaux de Paris (AP-HP, Greater Paris University Hospitals). Tocilizumab improves significantly clinical outcomes of patients with moderate or severe COVID-19 pneumonia Available at: https://www.aphp.fr/contenu/tocilizumab-improves-significantly-clinical-outcomes-patients-moderate-or-severe-covid-19. Accessed 27 April 2020.
- 37. Regeneron Pharmaceuticals, Inc. Regeneron and Sanofi provide update on U.S. phase 2/3 adaptive-designed trial of KEVZARA® (SARILUMAB) in hospitalized COVID-19 patients Available at: https://investor.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-provide-update-us-phase-23-adaptive. Accessed 27 April 2020.
- 38. Gritti G, Raimondi F, Ripamonti D, et al. . Use of siltuximab in patients with COVID-19 pneumonia requiring ventilatory support. 2020:2020.2004.2001.20048561. doi: 10.1101/2020.04.01.20048561. [DOI]
- 39. Mahmoudjafari Z, Hawks KG, Hsieh AA, et al. . American Society for Blood and Marrow Transplantation Pharmacy Special Interest Group Survey on chimeric antigen receptor T cell therapy administrative, logistic, and toxicity management practices in the United States. Biol Blood Marrow Transplant 2019; 25:26–33. [DOI] [PubMed] [Google Scholar]
- 40. Chen F, Teachey DT, Pequignot E, et al. . Measuring IL-6 and sIL-6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy. J Immunol Methods 2016; 434:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nishimoto N, Terao K, Mima T, et al. . Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 2008; 112:3959–64. [DOI] [PubMed] [Google Scholar]
- 42. ClinicalTrials.gov. Tocilizumab in COVID-19 pneumonia (TOCIVID-19) Available at: https://ClinicalTrials.gov/show/NCT04317092. Accessed 11 May 2020.
- 43. ClinicalTrials.gov. Tocilizumab to prevent clinical decompensation in hospitalized, non-critically ill patients with COVID-19 pneumonitis Available at: https://ClinicalTrials.gov/show/NCT04331795. Accessed 11 May 2020.
- 44. ClinicalTrials.gov. Clinical trial of combined use of hydroxychloroquine, azithromycin, and tocilizumab for the treatment of COVID-19 Available at: https://ClinicalTrials.gov/show/NCT04332094. Accessed 11 May 2020.
- 45. ClinicalTrials.gov. A study to evaluate the safety and efficacy of tocilizumab in patients with severe COVID-19 pneumonia Available at: https://ClinicalTrials.gov/show/NCT04320615. Accessed 11 May 2020.
- 46. ClinicalTrials.gov. Efficacy and safety of tocilizumab in the treatment of SARS-Cov-2 related pneumonia Available at: https://ClinicalTrials.gov/show/NCT04332913. Accessed 11 May 2020.
- 47. ClinicalTrials.gov. Tocilizumab vs CRRT in management of cytokine release syndrome (CRS) in COVID-19 Available at: https://ClinicalTrials.gov/show/NCT04306705. Accessed 11 May 2020.
- 48. ClinicalTrials.gov. Favipiravir combined with tocilizumab in the treatment of corona virus disease 2019 Available at: https://ClinicalTrials.gov/show/NCT04310228. Accessed 11 May 2020.
- 49. ClinicalTrials.gov. Prospective study in patients with advanced or metastatic cancer and SARS-CoV-2 (COVID-19) infection Available at: https://ClinicalTrials.gov/show/NCT04333914. Accessed 11 May 2020.
- 50. ClinicalTrials.gov. Cohort multiple randomized controlled trials open-label of immune modulatory drugs and other treatments in COVID-19 patients - tocilizumab trial - CORIMUNO-19 - TOCI (CORIMUNO-TOCI) Available at: https://ClinicalTrials.gov/show/NCT04331808. Accessed 11 May 2020.
- 51. ClinicalTrials.gov. Tocilizumab for SARS-CoV2 severe pneumonitis Available at: https://ClinicalTrials.gov/show/NCT04315480. Accessed 11 May 2020.
- 52. ClinicalTrials.gov. Treatment of COVID-19 patients with anti-interleukin drugs Available at: https://ClinicalTrials.gov/show/NCT04330638. Accessed 11 May 2020.
- 53. ClinicalTrials.gov. Anti-il6 treatment of serious COVID-19 disease with threatening respiratory failure Available at: https://ClinicalTrials.gov/show/NCT04322773. Accessed 11 May 2020.
- 54. ClinicalTrials.gov. Evaluation of the efficacy and safety of sarilumab in hospitalized patients with COVID-19 Available at: https://ClinicalTrials.gov/show/NCT04315298. Accessed 11 May 2020.
- 55. ClinicalTrials.gov. Sarilumab COVID-19 Available at: https://ClinicalTrials.gov/show/NCT04327388. Accessed 11 May 2020.
- 56. ClinicalTrials.gov. Cohort multiple randomized controlled trials open-label of immune modulatory drugs and other treatments in COVID-19 patients - sarilumab trial - CORIMUNO-19 - SARI Available at: https://ClinicalTrials.gov/show/NCT04324073. Accessed 11 May 2020.
- 57. ClinicalTrials.gov. Treatment of moderate to severe coronavirus disease (COVID-19) in hospitalized patients Available at: https://ClinicalTrials.gov/show/NCT04321993. Accessed 11 May 2020.
- 58. Grupp SA, Kalos M, Barrett D, et al. . Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368:1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Teachey DT, Rheingold SR, Maude SL, et al. . Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 2013; 121:5154–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pawar A, Desai RJ, Solomon DH, et al. . Risk of serious infections in tocilizumab versus other biologic drugs in patients with rheumatoid arthritis: a multidatabase cohort study. Ann Rheum Dis 2019; 78:456–64. [DOI] [PubMed] [Google Scholar]
- 61. Lamb YN, Deeks ED. Sarilumab: a review in moderate to severe rheumatoid arthritis. Drugs 2018; 78:929–40. [DOI] [PubMed] [Google Scholar]
- 62. Lee EB. A review of sarilumab for the treatment of rheumatoid arthritis. Immunotherapy 2018; 10:57–65. [DOI] [PubMed] [Google Scholar]
- 63. Emery P, Rondon J, Parrino J, et al. . Safety and tolerability of subcutaneous sarilumab and intravenous tocilizumab in patients with rheumatoid arthritis. Rheumatology (Oxford) 2019; 58:849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Burmester GR, Lin Y, Patel R, et al. . Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial. Ann Rheum Dis 2017; 76:840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Richardson P, Griffin I, Tucker C, et al. . Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020; 395:e30–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stebbing J, Phelan A, Griffin I, et al. . COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis 2020; 20:400–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sorrell FJ, Szklarz M, Abdul Azeez KR, et al. . Family-wide structural analysis of human numb-associated protein kinases. Structure 2016; 24:401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sutent (Sunitinib) [prescribing information]. New York, NY: Pfizer Laboratories; 2019. [Google Scholar]
- 69. Tarceva (Erlotinib) [prescribing information]. South San Francisco, CA: Genetech USA Inc; 2016. [Google Scholar]
- 70. Jakafi (Ruxolitinib) [prescribing information]. Wilmington, DE: Incyte Corporation; 2020. [Google Scholar]
- 71. Inrebic (Fedratinib) [prescribing information]. Summit, NJ: Celgene Corporation; 2019. [Google Scholar]
- 72. Olumiant (Baricitinib) [prescribing information]. Indianapolis, IN: Lilly USA LLC; 2019. [Google Scholar]
- 73. Pu SY, Xiao F, Schor S, et al. . Feasibility and biological rationale of repurposing sunitinib and erlotinib for dengue treatment. Antiviral Res 2018; 155:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect 2020; 53(3):368–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Praveen D, Puvvada RC, M VA. BARICITINIB - Janus kinase inhibitor baricitinib is not an ideal option for management of COVID-19. Int J Antimicrob Agents 2020; 55:105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. ClinicalTrials.gov. Baricitinib in symptomatic patients infected by COVID-19: an open-label, pilot study Available at: https://ClinicalTrials.gov/show/NCT04320277. Accessed 11 May 2020.
- 77. The Chinese Clinical Test Registration Center. Severe novel coronavirus pneumonia (COVID-19) patients treated with ruxolitinib in combination with mesenchymal stem cells: a prospective, single blind, randomized controlled clinical trial Available at: http://www.chictr.org.cn/showprojen.aspx?proj=49088. Accessed 11 May 2020.
- 78. ClinicalTrials.gov. Study of the efficacy and safety of ruxolitinib to treat COVID-19 pneumonia Available at: https://ClinicalTrials.gov/show/NCT04331665. Accessed 11 May 2020.
- 79. Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev 2018; 281:8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Park WY, Goodman RB, Steinberg KP, et al. . Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001; 164:1896–903. [DOI] [PubMed] [Google Scholar]
- 81. Kineret (Anakinra) [prescribing information]. Stockholm, Sweden: Swedish Orphan Biovitrum AB; 2018. [Google Scholar]
- 82. Opal SM, Fisher CJ Jr, Dhainaut JF, et al. . Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med 1997; 25:1115–24. [DOI] [PubMed] [Google Scholar]
- 83. Fisher CJ Jr, Dhainaut JF, Opal SM, et al. . Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA 1994; 271:1836–43. [PubMed] [Google Scholar]
- 84. Shakoory B, Carcillo JA, Chatham WW, et al. . Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med 2016; 44:275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Aouba A, Baldolli A, Geffray L, et al. . Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. [published online ahead of print May 6, 2020] Ann Rheum Dis 2020:annrheumdis-2020–217706. doi: 10.1136/annrheumdis-2020-217706. [DOI] [PubMed]
- 86. ClinicalTrials.gov. Efficacy and safety of emapalumab and anakinra in reducing hyperinflammation and respiratory distress in patients with COVID-19 infection Available at: https://ClinicalTrials.gov/show/NCT04324021. Accessed 11 May 2020.
- 87. Tuder RM, Yun JH. Vascular endothelial growth factor of the lung: friend or foe. Curr Opin Pharmacol 2008; 8:255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol 2006; 290:L209–21. [DOI] [PubMed] [Google Scholar]
- 89. Barratt S, Medford AR, Millar AB. Vascular endothelial growth factor in acute lung injury and acute respiratory distress syndrome. Respiration 2014; 87:329–42. [DOI] [PubMed] [Google Scholar]
- 90. Thickett DR, Armstrong L, Christie SJ, Millar AB. Vascular endothelial growth factor may contribute to increased vascular permeability in acute respiratory distress syndrome. Am J Respir Crit Care Med 2001; 164:1601–5. [DOI] [PubMed] [Google Scholar]
- 91. Watanabe M, Boyer JL, Crystal RG. Genetic delivery of bevacizumab to suppress vascular endothelial growth factor-induced high-permeability pulmonary edema. Hum Gene Ther 2009; 20:598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. ClinicalTrials.gov. Efficacy of bevacizumab in preventing acute respiratory distress syndrome (ARDS) Available at: https://ClinicalTrials.gov/show/NCT01314066. Accessed 11 May 2020.
- 93. ClinicalTrials.gov. Bevacizumab in severe or critically severe patients with COVID-19 pneumonia-RCT Available at: https://ClinicalTrials.gov/show/NCT04305106. Accessed 11 May 2020.
- 94. ClinicalTrials.gov. Bevacizumab in severe or critical patients with COVID-19 pneumonia Available at: https://ClinicalTrials.gov/show/NCT04275414. Accessed 11 May 2020.
- 95. Corne J, Chupp G, Lee CG, et al. . IL-13 stimulates vascular endothelial cell growth factor and protects against hyperoxic acute lung injury. J Clin Invest 2000; 106:783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Compernolle V, Brusselmans K, Acker T, et al. . Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med 2002; 8:702–10. [DOI] [PubMed] [Google Scholar]
- 97. Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol 2009; 6:465–77. [DOI] [PubMed] [Google Scholar]
- 98. Chen Z, Mi L, Xu J, et al. . Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J Infect Dis 2005; 191:755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wang K, Chen W, Zhou Y-S, et al. . SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. 2020:2020.2003.2014.988345. doi: 10.1101/2020.03.14.988345. [DOI]
- 100. ClinicalTrials.gov. Clinical study of anti-CD147 humanized meplazumab for injection to treat with 2019-nCoV pneumonia Available at: https://ClinicalTrials.gov/show/NCT04275245. Accessed 11 May 2020.
- 101. Tian RR, Zhang MX, Zhang LT, et al. . CD24 and Fc fusion protein protects SIVmac239-infected Chinese rhesus macaque against progression to AIDS. Antiviral Res 2018; 157:9–17. [DOI] [PubMed] [Google Scholar]
- 102. ClinicalTrials.gov. CD24Fc as a non-antiviral immunomodulator in COVID-19 treatment Available at: https://ClinicalTrials.gov/show/NCT04317040. Accessed 11 May 2020.
- 103. Weinblatt ME, Keystone EC, Furst DE, et al. . Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 2003; 48:35–45. [DOI] [PubMed] [Google Scholar]
- 104. Gong J, Dong H, Xia SQ, et al. . Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19 pneumonia. 2020:2020.2002.2025.20025643. doi: 10.1101/2020.02.25.20025643. [DOI] [PMC free article] [PubMed]
- 105. Humira (Adalimumab) [prescribing information]. North Chicago, IL: AbbVie Inc; 2020. [Google Scholar]
- 106. The Chinese Clinical Test Registration Center. A clinical study for the efficacy and safety of Adalimumab injection in the treatment of patients with severe novel coronavirus pneumonia (COVID-19) Available at: http://www.chictr.org.cn/showprojen.aspx?proj=49889. Accessed 11 May 2020.
- 107. The Chinese Clinical Test Registration Center. Efficacy and safety of tozumab combined with adamumab(Qletli) in severe and critical patients with novel coronavirus pneumonia (COVID-19) Available at: http://www.chictr.org.cn/showprojen.aspx?proj=50693. Accessed 11 May 2020.
- 108. Ying W, Qian Y, Kun Z. Drugs supply and pharmaceutical care management practices at a designated hospital during the COVID-19 epidemic. [published online ahead of print April 6, 2020] Res Social Adm Pharm 2020; S1551-7411(20):30325–9. doi: 10.1016/j.sapharm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Brigham and Women’s Hospital COVID-19 critical care clinical guidelines Available at: https://www.covidprotocols.org/. Accessed 31 March 2020.
- 110. NICE. National Institute for Health and Care Excellence. Coronavirus (COVID-19) Available at: https://www.nice.org.uk/covid-19. Accessed 31 March 2020.
- 111. UW Medicine. UW Medicine COVID-19 resource site - UW ID treatment guidelines Available at: https://covid-19.uwmedicine.org/Pages/default.aspx. Accessed 31 March 2020.
- 112. VUMC. Vanderbilt University Medical Center clinical recommendatoins for treatment of COVID-19 adult patients Available at: https://www.vumc.org/coronavirus/sites/default/files/COVID%20Documents/Weekly%20COVID-19%20Clinical%20Guidelines%20Update%20Summary%20-%203.22.2020-%20FINAL.pdf. Accessed 1 April 2020.
- 113. Wilson KC, Chotirmall SH, Bai C, Rello J.. On behalf of the International Task Force on COVID‐19. COVID‐19: Interim Guidance on Management Pending Empirical Evidence. From an American Thoracic Society‐led International Task Force. Available at: https://www.thoracic.org/covid/covid-19-guidance.pdf. Accessed 11 May 2020. [Google Scholar]
- 114. Alhazzani W, Møller MH, Belley-Cote E, Citerio G. Intensive care medicine rapid practice guidelines (ICM-RPG): paving the road of the future. Intensive Care Med 2019; 45:1639–41. [DOI] [PubMed] [Google Scholar]
- 115. Italian Society of Infectious and Tropical Diseases: Handbook for the Care of People with Disease-COVI 19. 2nd ed. Available at: http://www.simit.org/IT/simit/sezioni-regionali.xhtml/sezione/112-lombardia/comunicazioni/1. Accessed 31 March 2020. [Google Scholar]
- 116. Van Ierssel S, Dauby N, Bottieau E, et al. . Interim clinical guidance for adults with suspect or confirmed COVID -19 in Belgium Available at: https://epidemio.wiv-isp.be/ID/Documents/Covid19/COVID-19_InterimGuidelines_Treatment_ENG.pdf. Accessed 31 March 2020.
- 117. University of Michigan: Michigan Medicine Univeristy of Michigan inpatient guidance for treatment of COVID-19 in adults and children Available at: http://www.med.umich.edu/asp/pdf/adult_guidelines/COVID-19-treatment.pdf. Accessed 31 March 2020.
- 118. University of Pennsylvania Health System - Penn Medicine treatment guidelines for SARS-CoV-2 infection - treatment of adult patients with confirmed SARS-CoV-2 (COVID-19) infection Available at: http://www.uphs.upenn.edu/antibiotics/COVID19.html. Accessed 31 March 2020.
- 119. Auwaerter PG. John Hopkins ABX guide Coronavirus Covid-19 (SARS-CoV-2) Available at: https://www.hopkinsguides.com/hopkins/view/Johns_Hopkins_ABX_Guide/540747/all/Coronavirus_COVID_19__SARS_CoV_2_. Accessed 1 April 2020.
- 120. Massachusetts General Hospital COVID-19 treatment guidance Available at: https://www.massgeneral.org/assets/MGH/pdf/news/coronavirus/MGH%20ID%20COVID19%20Here%20and%20Now%20Treatment%20Guidance%20V1.351%2003272020.pdf. Accessed 1 April 2020.
- 121. Yale New Haven Health System initial treatment algorithm for patients with COVID-19 Available at: https://files-profile.medicine.yale.edu/documents/e91b4e5c-ae56-4bf1-8d5f-e674b6450847. Accessed 11 May 2020.
- 122. University of Mississippi Medical Center. Univeristy of Mississippi Medical Center Management of patients with suspected SARS-CoV-2 (COVID-19) Available at: https://www.umc.edu/CoronaVirus/Mississippi-Health-Care-Professionals/Clinical-Resources/Patient%20Treatment%20Guidelines/Management%20of%20Patients.html. Accessed 11 May 2020.
- 123. Mount Sinai Health System treatment guidelines for SARS-CoV-2 infection (COVID-19) Available at: https://www.mountsinai.org/files/MSHealth/Assets/HS/About/Coronavirus/MSHS-Treatment-Guidelines-COVID.pdf. Accessed 11 May 2020.
- 124. AMITA Health COVID-19 treatment guidelines Available at: https://www.amitahealth.org/assets/documents/covid-19-playbook/amita-health-covid-19-treatment-guidelines-4-11-2020.pdf. Accessed 11 May 2020.
- 125. University of Iowa Health Care COVID-19 clinical information Available at: https://medcom.uiowa.edu/theloop/covid-19-clinical-information#covid-19-treatment-guide. Accessed 11 May 2020.
- 126. Alexander B, Van Schooneveld T, Stohs E, et al. . Nebraska Medicine Covid-19 antiviral and pharmacotherapy information Available at: https://www.nebraskamed.com/sites/default/files/documents/covid-19/antiviral-and-pharmacotherapy-information.pdf?date=03242020. Accessed 1 April 2020.
- 127. Avastin (Bevacizumab) [prescribing information]. South San Francisco, CA: Genentech; 2019. [Google Scholar]
- 128. Bian H, Zheng Z-H, Wei D, et al. . Meplazumab treats COVID-19 pneumonia: an open-labelled, concurrent controlled add-on clinical trial. 2020:2020.2003.2021.20040691. doi: 10.1101/2020.03.21.20040691. [DOI]