Abstract
Background
Coronavirus disease 2019 (COVID-19) is an emerging infectious disease that first manifested in humans in Wuhan, Hubei Province, China, in December 2019, and has subsequently spread worldwide.
Methods
We conducted a retrospective, single-center case series of the seven maintenance hemodialysis (HD) patients infected with COVID-19 at Zhongnan Hospital of Wuhan University from 13 January to 7 April 2020 and a proactive search of potential cases by chest computed tomography (CT) scans.
Results
Of 202 HD patients, 7 (3.5%) were diagnosed with COVID-19. Five were diagnosed by reverse transcription polymerase chain reaction (RT-PCR) because of compatible symptoms, while two were diagnosed by RT-PCR as a result of screening 197 HD patients without respiratory symptoms by chest CT. Thirteen of 197 patients had positive chest CT features and, of these, 2 (15%) were confirmed to have COVID-19. In COVID-19 patients, the most common features at admission were fatigue, fever and diarrhea [5/7 (71%) had all these]. Common laboratory features included lymphocytopenia [6/7 (86%)], elevated lactate dehydrogenase [3/4 (75%)], D-dimer [5/6 (83%)], high-sensitivity C-reactive protein [4/4 (100%)] and procalcitonin [5/5 (100%)]. Chest CT showed bilateral patchy shadows or ground-glass opacity in the lungs of all patients. Four of seven (57%) received oxygen therapy, one (14%) received noninvasive and invasive mechanical ventilation, five (71%) received antiviral and antibacterial drugs, three (43%) recieved glucocorticoid therapy and one (14%) received continuous renal replacement therapy. As the last follow-up, four of the seven patients (57%) had been discharged and three patients were dead.
Conclusions
Chest CT may identify COVID-19 patients without clear symptoms, but the specificity is low. The mortality of COVID-19 patients on HD was high.
Keywords: COVID-19, chronic renal failure, clinical outcome, hemodialysis, SARS-CoV-2
INTRODUCTION
In December 2019, a cluster of acute respiratory illness occurred in Wuhan, Hubei Province, China [1–3]. The pathogen was identified as a novel enveloped ribonucleic acid (RNA) beta coronavirus that has been named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which has a phylogenetic similarity to SARS-CoV [4, 5]. The World Health Organization (WHO) has named the disease coronavirus disease 2019 (COVID-19). COVID-19 is more contagious than SARS and often results in acute respiratory distress syndrome (ARDS), spreads by human-to-human transmission via droplets or direct contact and has an incubation period estimated at 1–14 days (usually 3–7 days) [6]. On 11 March 2020, the WHO declared COVID-19 a pandemic.
This study evaluates the impact of chest computed tomography (CT) screening of hemodialysis (HD) patients without respiratory symptoms as well as the clinical, laboratory and radiologic features and outcomes of HD patients with COVID-19.
MATERIALS AND METHODS
Study design and patients
We did a retrospective review of medical records from HD patients infected with COVID-19 at Zhongnan Hospital of Wuhan University from 12 January to 7 April 2020. The diagnosis of COVID-19 pneumonia was based on the New Coronavirus Pneumonia Prevention and Control Program (4th edition) published by the National Health Commission of China [6] and was based on testing positive for SARS-CoV-2 by use of quantitative reverse transcription polymerase chain reaction (qRT-PCR) on respiratory tract samples. This case series was approved by the institutional ethics board of Zhongnan Hospital of Wuhan University (no. 2020015). The clinical outcomes were monitored up to 7 April 2020.
Data collection
The medical records of patients were analyzed by the research team of the Department of Nephrology, Radiology and Respiratory Medicine. Epidemiological, clinical, laboratory and radiological characteristics and treatment and outcome data were obtained from electronic medical records. The data were reviewed by a trained team of physicians. Information recorded included demographic data, medical history, exposure history, symptoms, signs, complications, laboratory findings, chest CT scans and treatment measures (i.e. antiviral therapy, corticosteroid therapy and respiratory support).
Real-time RT-PCR assay for SARS-CoV-2
Throat swab samples were collected and tested for SARS-CoV-2 with the Chinese Center for Disease Control and Prevention (CDC)-recommended kit (BioGerm, Shanghai, China), following WHO guidelines for RT-PCR [7–9]. All samples were processed at the Department of Clinical Laboratory. The test results were confirmed by nested RT-PCR with designed primers. Positive cases of COVID-19 infection were defined from the RT-PCR results.
Statistical analysis
Statistical analysis was done with SPSS version 20.0 (IBM, Armonk, NY, USA). Continuous variables were directly expressed as a range and categorical variables were expressed as number (%).
RESULTS
Diagnosis of COVID-19 in HD patients
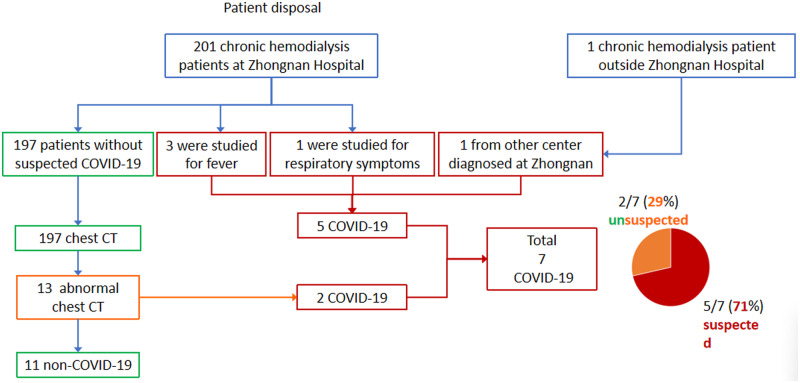
A total of 202 patients had been on HD twice or three times weekly in the dialysis center in Zhongnan Hospital. The number of weekly sessions did not change because of the epidemic. The COVID-19 patients were identified as follows (Figure 1):
FIGURE 1.
The disposal flow chart for 202 patients.
Three patients (patients 3, 5 and 6) with fever were confirmed COVID-19 on fever clinics.
One patient with respiratory symptoms was suspected to have COVID-19 and this was later confirmed. These four patients were admitted to the hospital treatment.
One further patient from another dialysis center was confirmed COVID-19 after admission in our hospital. These five patients received HD in our center during the incubation period.
One hundred ninety-seven HD patients without fever and respiratory symptoms had blood cell count and chest CT examination. We determined the presence of a CT abnormality on the basis of the imaging scans. They were reviewed by attending physicians in respiratory medicine and radiology who extracted the data. They suggested patients with positive chest CT features must have a CT twice and test SARS-CoV-2 three times. Patients could continue to receive HD in our center if SARS-CoV-2 was negative and CT features showed absorption or no change. Thirteen patients had positive chest CT features. CT images showed ground-glass opacity in the lungs (Figure 2). Two of 13 patients (15%) were confirmed to have COVID-19 (patients 4 and 7). In 11 patients, chest CT and SARS-CoV-2 were repeated after 1 week. CT images showed the absorption of ground glass in 10 patients and no change in 1 patient (Figure 2); none were positive for SARS-CoV-2 (Table 1).
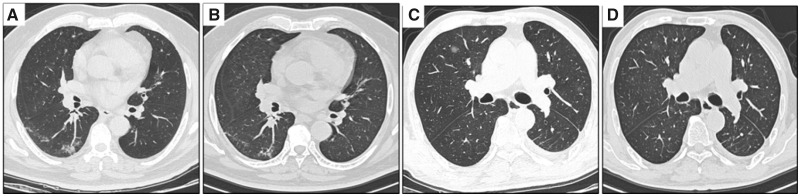
FIGURE 2.
Chest CT scans (transverse plane) of patients without COVID-19. (A) Patient 1: patchy ground-glass opacities located in the subpleural area of right lower lung lobe. (B) Patient 1: the opacities were absorbed a little after 1 week. (C) Patient 2: round ground-glass opacity in the right upper lung lobe with a clear border. (D) Patient 2: the opacity was much absorbed after 1 week.
Table 1.
Laboratory and chest CT findings in patients without respiratory symptoms
| Findings | First chest CT197 patients | First RT-PCR13 patients | Second RT-PCR11 patients(after 24 hours) | Second chest CT11 patients(after 1 week) | Third RT-PCR11 patients (after 1 week) |
|---|---|---|---|---|---|
| Chest CT images | |||||
| No CT abnormality | 184 | – | – | 10 | – |
| Ground-glass opacity | 13 | – | – | 1 | – |
| SARS-CoV-2 RT-PCR | |||||
| Negative | – | 11 | 11 | – | 11 |
| Positive | – | 2 | 0 | – | 0 |
Clinical features of patients with COVID-19
All patients had a history of epidemiological exposure to COVID-19. Seven HD patients were infected with SARS-CoV-2 and confirmed to have COVID-19. The age range was 47–67 years, HD vintage 1–7 years and four were men (Table 2). Clinical characteristics are shown in Table 2. The most common symptoms were fatigue, fever and diarrhea [five of seven cases (71%)]. Four of seven cases (57%) had dyspnea. Cough and anorexia were reported in three cases (43%).
Table 2.
Baseline characteristics of seven patients infected with COVID-19
| Findings | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Abnormal n/total N (%) |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 66 | 62 | 61 | 62 | 47 | 67 | 51 | |
| Sex | Male | Female | Male | Male | Female | Female | Male | |
| Epidemiological history | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7/7 (100) |
| Underlying diseases | Hypertension | Lupus nephritis | Hypertension | Hypertension | Chronic nephritis | Hypertension | Hypertension | |
| Dialysis vintage (years) | 3 | 6 | 7 | 3 | 5 | 1 | 1 | |
| Signs and symptoms | ||||||||
| Fever | Yes | Yes | Yes | No | Yes | Yes | No | 5/7 (71) |
| Fatigue | Yes | Yes | Yes | No | No | Yes | Yes | 5/7 (71) |
| Cough | Yes | Yes | No | Yes | No | No | No | 3/7 (43) |
| Expectoration | Yes | No | No | No | No | No | No | 1/7 (14) |
| Dyspnea | Yes | Yes | No | No | Yes | Yes | No | 4/7 (57) |
| Anorexia | Yes | Yes | No | No | No | No | Yes | 3/7 (43) |
| Nausea | Yes | No | No | No | No | No | No | 1/7 (14) |
| Vomit | No | Yes | No | No | No | No | No | 1/7 (14) |
| Diarrhea | No | Yes | Yes | No | Yes | Yes | Yes | 5/7 (71) |
| Diagnosis triggered by | Symptoms | Symptoms | Symptoms | Chest CT | Symptoms | Symptoms | Chest CT | |
| Outcome | Death | Discharge | Discharge | Death | Discharge | Discharge | Death | |
Common laboratory features included lymphocytopenia [6/7 (86%)], elevated lactate dehydrogenase [3/4 (75%)], D-dimer [5/6 (83%)], high-sensitivity C-reactive protein [4/4 (100%)] and procalcitonin [5/5 (100%)]. Less common laboratory features include leukopenia [1/7 (14%)], thrombocytopenia [1/7 (14%)] and elevated aspartate aminotransferase [1/6 (17%)]. Creatine kinase and creatine kinase-MB were normal in six patients (Table 3). All seven patients underwent chest CT and had findings consistent with pneumonia (Figure 3). Bilateral ground-glass opacities and consolidation were the most common radiologic findings (Table 3; Figure 3).
Table 3.
Laboratory characteristics, complications and treatments of seven COVID-19 patients
| Laboratory characteristics | Normal range | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Abnormal n/total N (%) |
|---|---|---|---|---|---|---|---|---|---|
| White blood cell count (× 109/L) | 3.5–9.5 | 2.69 | 8.02 | 6.84 | 7.5 | 7.73 | 10.76 | 5.03 | 2/7 (29) |
| Neutrophil count (× 109/L) | 1.8–6.3 | 2.24 | 5.52 | 5.69 | 5.65 | 6.28 | 9.24 | 4.29 | 1/7 (14) |
| Lymphocyte count (× 109/L) | 1.1–3.2 | 0.22 | 1.97 | 0.63 | 0.84 | 0.80 | 0.92 | 0.49 | 6/7 (86) |
| Platelet count (× 109/L) | 125–350 | 97 | 311 | 122 | 200 | 114 | 213 | 141 | 1/7 (14) |
| Activated partial thromboplastin time (sec) | 25.1–36.5 | 39.1 | 29.5 | 57.3 | 28.7 | 70.1 | 32.4 | NA | 3/6 (50) |
| D-dimer (mg/L) | 0–500 | 2862 | 650 | 1210 | 1208 | 1240 | 450 | NA | 5/6 (83) |
| Creatine kinase (U/L) | <171 | 67 | 126 | NA | NA | 73 | 84 | NA | 0/4 (0) |
| Creatine kinase-MB (U/L) | 0–25 | 16 | 20 | 13 | 12 | 22 | 14 | NA | 0/6 (0) |
| Lactate dehydrogenase (U/L) | 125–243 | 277 | NA | 190 | NA | 308 | 321 | NA | 3/4 (75) |
| High-sensitivity C-reactive protein concentration (mg/L) | 0–3 | NA | 3.13 | 35.3 | NA | 10 | 176.5 | NA | 4/4 (100) |
| Alanine aminotransferase (U/L) | 9–50 | 38 | 11 | 11 | 17 | 10 | 17 | NA | 0/6 (0) |
| Aspartate aminotransferase (U/L) | 15–40 | 66 | 32 | 18 | 24 | 8 | 25 | NA | 1/6 (17) |
| Procalcitonin (ng/mL) | <0.05 | 6.57 | 0.35 | NA | 7.13 | 1.86 | 1.12 | NA | 5/5 (100) |
| Chest CT images | |||||||||
| Bilateral lung disease | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7/7 (100) | |
| Complications | |||||||||
| Acute cardiac injury | No | No | No | No | No | No | No | 0/7 (0) | |
| ARDS | Yes | No | No | No | No | No | No | 1/7 (14) | |
| Shock | Yes | No | No | No | No | No | No | 1/7 (14) | |
| Treatment | |||||||||
| Antiviral therapy | Yes | Yes | Yes | No | Yes | Yes | No | 5/7 (71) | |
| Antibacterial therapy | Yes | Yes | Yes | No | Yes | Yes | No | 5/7 (71) | |
| Glucocorticoid therapy | Yes | No | No | No | Yes | Yes | No | 3/7 (43) | |
| CRRT | Yes | No | No | No | No | No | No | 1/7 (14) | |
| Oxygen inhalation | Yes | Yes | No | No | Yes | Yes | No | 4/7 (57) | |
| NIV | Yes | No | No | No | No | No | No | 1/7 (14) | |
| IMV | Yes | No | No | No | No | No | No | 1/7 (14) |
Acute cardiac injury: serum levels of cardiac biomarkers (e.g. troponin I) above the 99th percentile upper reference limit; NIV, noninvasive ventilation; IMV, invasive mechanical ventilation.
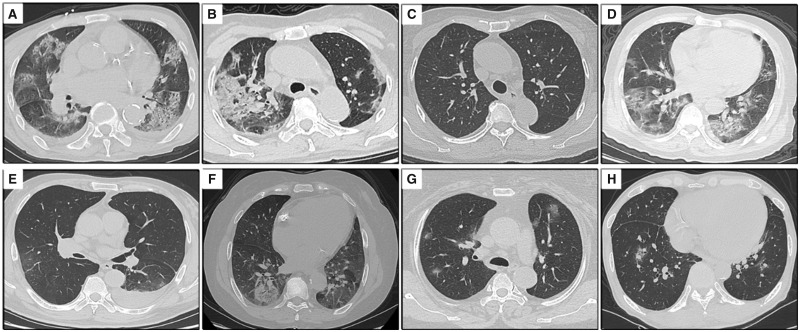
FIGURE 3.
Chest CT scans (transverse plane) of seven patients with COVID-19. (A) Patient 1: bilateral multiple consolidations and ground-glass opacities. (B) Patient 2: patchy consolidation in the right lung and bilateral ground-glass opacities. (C) Patient 2: bilateral lesions absorbed after 31 days. (D) Patient 3: bilateral multiple ground-glass opacities and a few consolidation opacities in the left lower lung lobe. (E) Patient 4: multiple ground-glass opacities bilaterally and left pleural effusion. (F) Patient 5: round mixed ground-glass opacity in the subpleural area of right lower lung lobe. (G) Patient 6: bilateral patchy ground-glass opacities. (H) Patient 7: bilateral patchy ground-glass opacities.
Five patients (71%) received antiviral drugs (ribavirin or arbidol; Table 3). Five patients (71%) also received antibiotic therapy after admission. Commonly used antibiotics included fluoroquinolones, meropenem and cephalosporins. Three patients (43%) were treated with at least one glucocorticoid, with the administration of intravenous methylprednisolone. Four patients (57%) received oxygen therapy. One patient was admitted to the intensive care unit (ICU) and required continuous renal replacement therapy (CRRT), noninvasive ventilation and invasive mechanical ventilation. One patient had a severe complication of ARDS and shock. As of 7 April, three patients had died (patients 1, 3 and 7). The causes of death were directly related to ARDS (n = 1, patient 1) and hyperkalemia (n = 2, patients 3 and 7). Four patients were discharged from the hospital with full recovery.
Infection control
All patients who visited HD clinics had a physical examination, blood cell count and chest CT. Patients with a normal body temperature can be treated at the designated dialysis machine and patients with an abnormal body temperature can be triaged according to fever clinics. All patients with fever should be screened for novel coronavirus infection and should be given dialysis during the last shift of the day until infection is excluded. If a new confirmed or highly suspected patient in a dialysis centre is identified, disinfection should be carried out immediately. Areas in close contact with these patients should not be used for other patients until cleared. The medical waste from confirmed or suspected patients with COVID-19 should be considered as infectious medical waste and disposed of accordingly.
CRRT was performed in a room inside the isolation ward designated for patients with COVID-19. Dialysis unit staff wore full protective gear, including waterproof disposable gown, cap, gloves, face shield and N95 face mask. Patients must wear surgical masks in the blood purification center. Dialysis filters and extracorporeal circulation tubes were discarded as infectious waste. Unused dialysate concentrates and sodium bicarbonate filters were also discarded. After each HD, the dialyzer was sterilized by heating 50% citric acid disinfectant to 85°C and circulating for 15 min according to the manufacturer’s instructions.
During the outbreak, seven HD patients had confirmed COVID-19. Patient 1 was treated with CRRT during hospitalization. The 11 patients with positive chest CT features but negative PCR were quarantined in two districts for 2 weeks. As of 7 April, no new patient had become infected with COVID-19.
DISCUSSION
COVID-19 is a newly discovered contagious disease caused by SARS-CoV-2, primarily manifesting as an acute respiratory illness with interstitial and alveolar pneumonia, but it can affect multiple organs, including the kidneys, heart, digestive tract, blood and nervous system [9]. Pregnant women, newborns and the elderly and patients with comorbidities such as diabetes mellitus, hypertension and cardiovascular disease are susceptible to COVID-19 infection and likely to have more severe illness often requiring ICU care. HD patients are particularly susceptible to respiratory pathogens and severe pneumonia. This is probably related to the relative suppressed immunity of uremic patients and the frequent hospital admissions of patients on dialysis. For example, dialysis patients have a higher rate of contracting SARS compared with the general population [10].
We reported five confirmed COVID-19 HD patients through 13 February. We did not report clinical endpoints because these patients were still hospitalized at the time of manuscript submission [11]. We describe clinical data and outcomes from seven HD patients with laboratory-confirmed COVID-19 as of 7 April. The clinical characteristics of these patients with COVID-19 infection during HD were similar to those of non-HD adults with COVID-19 infection [12–14]. At presentation, patients on HD exhibit similar symptoms (fever, cough and fatigue) as nondialysis patients; however, most patients presented with diarrhea, which is different from nondialysis patients. Gastrointestinal symptoms may be difficult to distinguish from uremic symptoms. Only one patient presented initially with atypical symptoms such as nausea and vomiting.
In line with recent studies, lymphopenia was common [12–14]. On admission, lymphocytopenia was present in 86% of the patients, thrombocytopenia in 14% and leukopenia in 14%. Most patients had elevated levels of procalcitonin, high-sensitivity C-reactive protein, lactate dehydrogenase and D-dimer. Less common was elevated levels of aspartate aminotransferase. Bilateral distribution of patchy shadows and ground-glass opacity was a typical hallmark of CT scans for COVID-19.
In this case series, mortality was high [3/7 patients (43%)]. There is no specific effective antiviral drug for COVID-19 at present. CRRT has been successfully applied in the treatment of SARS, Middle East respiratory syndrome and sepsis [15, 16]. High-volume hemofiltration (HVHF) removed inflammatory cytokines [17]. Therefore CRRT and HVHF may play a role in patients with COVID-19. The potential role of extracorporeal therapy needs to be evaluated.
During the first 2 months of the current outbreak, all HD patients received HD twice or three times weekly in HD centers, as is routine in China. Patients with fever were confirmed as COVID-19 on fever clinics, but these patients received HD in an HD center during the incubation period. Chest CT examination is helpful in diagnosing COVID-19. Ground-glass opacities and consolidation in the lung periphery have been the imaging hallmark in patients with COVID-19 infection. Chest X-ray may not demonstrate the peripheral predominance that was visible on respective CT examinations, thus chest X-ray may lack the sensitivity to identify some of the manifestations of the COVID-19 infection in the lungs, which are otherwise evident on CT [18]. All patients without fever and respiratory symptoms and asymptomatic patients had blood cell counts and chest CT examinations in our HD center. Suspicion of COVID-19 by chest CT was only confirmed in 15% of these patients. We suggest that all HD patients have a chest CT examination to prevent missing patients infected with COVID-19.
COVID-19 infection presents particular challenges for patients on dialysis. We developed guidelines for dialysis units during the COVID-19 outbreak. All patients with fever should be screened for coronavirus infection and should be given dialysis during the last shift of the day until infection is excluded. Strict patient surveillance and proper isolation practices prevented secondary transmissions and HD could be performed safely.
The limitations of this study include the small sample size and its restrictive nature. Additionally, the lack of antibody evaluation means that some patients with past COVID-19 infection may not have been diagnosed.
In summary, in this single-center case series of 202 HD patients in Wuhan, China, COVID-19 was confirmed in 3.5% of patients. Many patients presented with diarrhea, which was different from nondialysis patients. Surprisingly, COVID-19 was only suspected because of classical symptoms in 71% of diagnosed patients, while 29% were diagnosed following screening of all HD patients with chest CT examination. Chest CT had low specificity (15%) for the identification of COVID-19 patients. Overall, mortality of COVID-19 in HD was high.
AUTHORS’ CONTRIBUTIONS
H.S. and M.L. designed the study. H.H., C.L. and H.H. collected data. Z.C., P.G, X.W., M.Y.L. and H.S. analyzed the data. R.W. and H.H. made the figures. R.W., H.H., C.L., M.L. and H.S. drafted and revised the manuscript. All the authors approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
All the authors declare that they have no relevant financial interests. The results presented in this article have not been published previously in whole or part.
REFERENCES
- 1. Lu H, Stratton CW, Tang YW.. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol 2020; 92: 401–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paules CI, Marston HD, Fauci AS.. Coronavirus infections-more than just the common cold. JAMA 2020; 323: 707–708 [DOI] [PubMed] [Google Scholar]
- 3.Wuhan Municipal Health Commission. Report of Cluster in Pneumonia of Unknown Etiology in Wuhan City, 2019. http://www.wuhan.gov.cn/front/web/showDetail/2019123108989 (31 January 2020, date last accessed)
- 4. Lu R, Zhao X, Li J. et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu N, Zhang D, Wang W. et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Health Commission of China. New Coronavirus Pneumonia Prevention And Control Program, 4th edn, 2020. http://www.gov.cn/zhengce/zhengceku/2020-01/28/5472673/files/0f96c10cc09d4d 36a6f9a9f0b42d972b (4 February 2020, date last accessed)
- 7.World Health Organization. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection Is Suspected, Interim guidance, 2020. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf (4 February 2020, date last accessed)
- 8. Corman VM, Landt O, Kaiser M. et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020; 25: doi:10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang D, Hu B, Hu C. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan. JAMA 2020; 323: 1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kwan BC, Leung CB, Szeto CC. et al. Severe acute respiratory syndrome in dialysis patients. J Am Soc Nephrol 2004; 15: 1883–1888 [DOI] [PubMed] [Google Scholar]
- 11. Wang R, Liao C, He H. et al. COVID-19 in hemodialysis patients: a report of 5 cases. Am J Kidney Dis 2020; doi:10.1053/j.ajkd.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang C, Wang Y, Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Q, Guan X, Wu P. et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med 2020; 382: 1199–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen N, Zhou M, Dong X. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chu KH, Tsang WK, Tang CS. et al. Acute renal impairment in coronavirus associated severe acute respiratory syndrome. Kidney Int 2005; 67: 698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arabi YM, Arifi AA, Balkhy HH. et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med 2014; 160: 389–397 [DOI] [PubMed] [Google Scholar]
- 17. Ghani RA, Zainudin S, Ctkong N. et al. Serum IL-6 and IL-1-ra with sequential organ failure assessment scores in septic patients receiving high-volume haemofiltration and continuous venovenous haemofiltration. Nephrology (Carlton) 2006; 11: 386–393 [DOI] [PubMed] [Google Scholar]
- 18. Wong HYF, Lam HYS, Fong AH. et al. Frequency and distribution of chest radiographic finding and distribution in COVID-19 positive patients. Radiology 2019; doi:10.1148/radiol.2020201160 [DOI] [PMC free article] [PubMed] [Google Scholar]