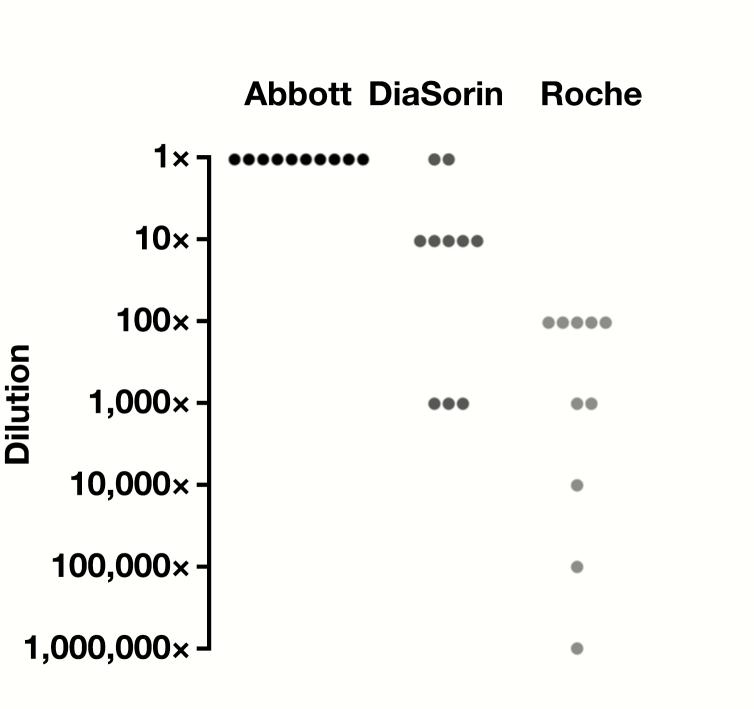
Figure 1.
Relative limits of detection of Abbott ID NOW, DiaSorin Simplexa, and Roche cobas using 10 nasopharyngeal swab–positive patient samples collected in universal viral transport medium. A 10-fold dilution series was made for each patient sample and tested on all 3 instruments until a negative result was obtained. For each patient series, results were normalized to the lowest detected dilution of Abbott ID NOW (1×) and the last detectable reaction below that dilution is indicated for each instrument. Each dot represents the lowest detected dilution for each patient relative to Abbott ID NOW. All data used for this analysis can be found in the supplemental dataset (all supplemental material is available at American Journal of Clinical Pathology online).