Abstract
Objectives:
To compare the clinical outcome of mechanically ventilated patients with severe acute respiratory syndrome coronavirus 2-related acute respiratory distress syndrome, who received corticosteroid with those who did not.
Design:
Retrospective analysis.
Setting:
Intensive care setting.
Patients:
All adult mechanically ventilated patients, who were admitted to the ICU between March 20, 2020, and May 10, 2020, for severe acute respiratory syndrome coronavirus 2-related acute respiratory distress syndrome.
Interventions:
None.
Measurements and Main Results:
Cohort was divided into two groups based on corticosteroid administration. The primary outcome variable was ventilator-free days at day 28. Secondary outcome variable was ICU-free days at day 30, and hospital-free days at day 30. Consecutive 61 mechanically ventilated patients with severe acute respiratory syndrome coronavirus 2-related acute respiratory distress syndrome were analyzed. Patient in corticosteroid group as compared with noncorticosteroid group have higher 28-day ventilator-free days (mean, 10.2; median, 7 [interquartile range, 0–22.3] vs mean, 4.7; median, 0 [interquartile range, 0–11]; p = 0.01).There was no significant difference noted in secondary outcomes (ICU-free days at day 30 and hospital-free days at day 30).
Conclusions:
Among mechanically ventilated severe acute respiratory syndrome coronavirus 2-related acute respiratory distress syndrome patients, corticosteroids use was associated with significant improvement in 28-day ventilator-free days at day 28, but no significant improvement in ICU-free days at day 30, and hospital-free days at day 30.
Keywords: acute respiratory distress syndrome, corticosteroids, mechanical ventilator, outcome, severe acute respiratory syndrome coronavirus 2
To the Editor:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has caused a global pandemic affecting over 4.5 million people as of May 15, 2020 (1). Acute respiratory distress syndrome (ARDS) has been reported in 5% of SARS-CoV-2 infected cases (2) and is associated with increased duration of mechanical ventilation and mortality (3, 4). There is no proven therapy for SARS-CoV-2-related ARDS, and the use of corticosteroids is controversial (5). The World Health Organization recommend against the routine use of steroids in patients SARS-CoV-2 pneumonia (6), yet the Society of Critical Care Medicine recently issued a weak recommendation supporting the use of corticosteroids in patients with SARS-CoV-2-related ARDS (7). There is no study to date, which has so far addressed the effect of corticosteroids administration on clinical outcome in mechanically ventilated patients with SARS-CoV-2-related ARDS.
This is a retrospective analysis of adult, mechanically ventilated patients, who were admitted to the ICU between March 20, 2020, and April 17, 2020, for SARS-CoV-2-related ARDS. This study was approved by the Institutional Review Board (IRB) at Albany Medical Center (IRB number 5826). SARS-CoV-2 infection was diagnosed via real-time reverse transcription-polymerase chain reaction from a nasopharyngeal swab in these patients. ARDS was defined by the Berlin criteria. The cohort was divided into two groups based on corticosteroid administration. The decision to administer corticosteroids was made by the treating clinician; some clinicians were routinely using steroid early in the course after excluding coinfection with other virus or bacterial infection and others were not.
The primary outcome variable was ventilator-free days at day 28. This is a composite outcome that aggregates length of mechanical ventilation and survival. Patients who remain on mechanical ventilation longer than 28 days or who die while on mechanical ventilation are given zero free days. Secondary outcome variable was ICU-free days at day 30 and hospital length of stay at day 30. To validate the association between the main covariable (steroids administration) associated with the outcome measures, we determined baseline similarity in terms of chronic disease (Charlson comorbidity index) and acute disease burden (Acute Physiology and Chronic Health Evaluation II, Sequential Organ Failure Assessment, and Simplified Acute Physiology Score II) between the groups. Minitab statistical software was used for analysis. Continuous variables were expressed as mean, median, and interquartile range (interquartile range [IQR], 25–75th percentile). For normally distributed data-independent sample t tests were used to assess statistical significance (two-tailed alpha of 0.05). For data not demonstrating normality, including the primary outcome variable, statistical significance was assessed using Mann-Whitney U nonparametric test. Categorical variables are expressed as numbers and percentages and statistical significance assessed using Pearson chi-square test or Fisher exact test when expected values are less than five.
We analyzed 61 mechanically ventilated patients with SARS-CoV-2-related ARDS. Study cohort had mean age of 60.2 years and were predominantly male (66%); Caucasians (28%) and Hispanics (28%) were the predominant ethnicity in the cohort. Corticosteroids were administered in 38 patients. Most common corticosteroid used was dexamethasone 36 of 38 (95%) followed by methylprednisolone two of 38 (5%). Average cumulative dose of dexamethasone and methylprednisolone was 143 mg and 120 mg, respectively, and mean duration was 9.2 days. There was no significant difference in baseline characteristics, severity score, and treatment received between corticosteroid group and noncorticosteroid group (Table 1).
TABLE 1.
Comparison of Baseline Characteristics and Outcomes Between Corticosteroid Versus Noncorticosteroid Groups
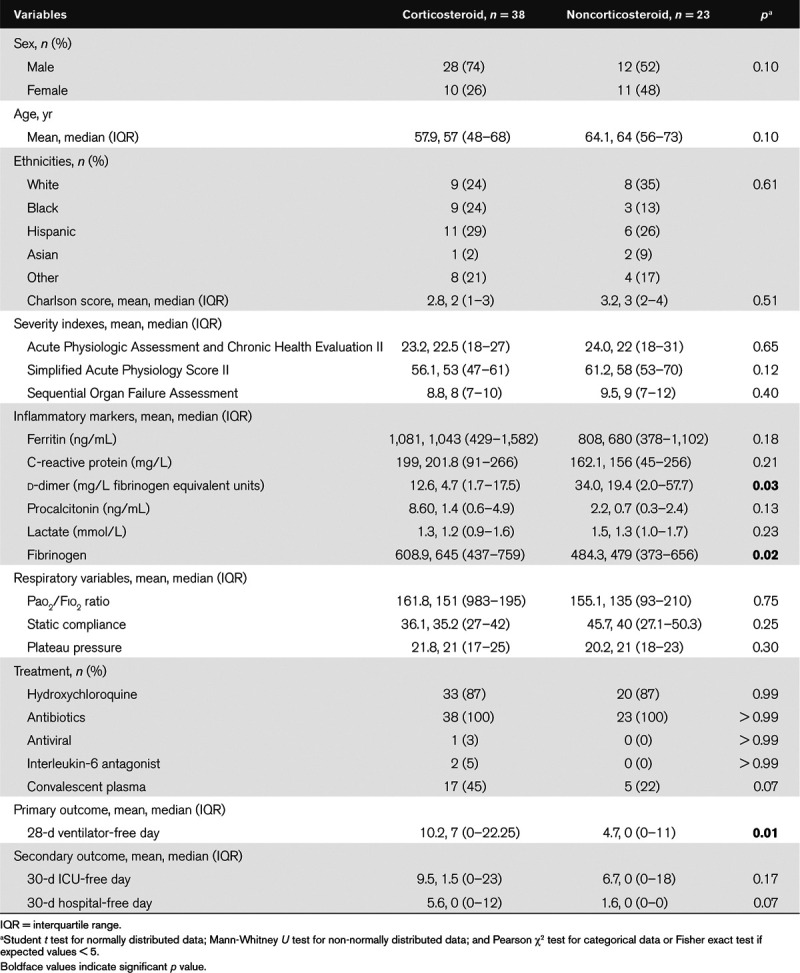
Patient in corticosteroid group as compared with noncorticosteroid group had higher mean 28 ventilation-free days (10.2; median, 7 [IQR, 0–22.3] vs 4.7; median, 0 [IQR, 0–11]; p = 0.01; Mann-Whitney U test) (Table 1 and Fig. 1). There was no significant difference noted in secondary outcomes (30-d hospital-free days or 30-d ICU-free days).
Figure 1.
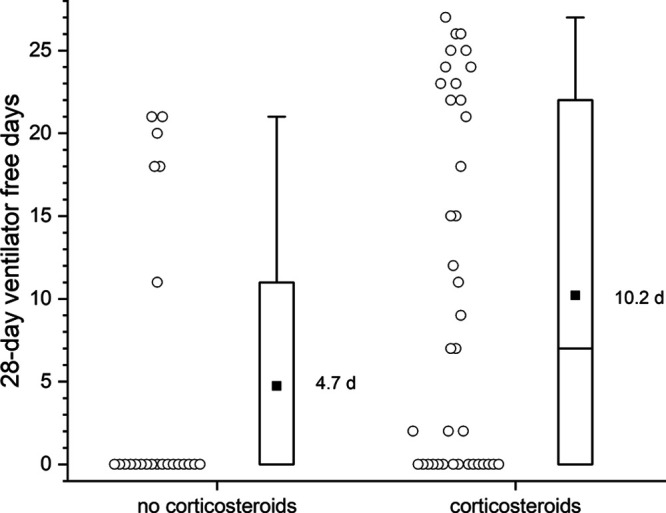
Dot plot showing the 28-d ventilator-free days between corticosteroid and noncorticosteroid group. Each circle indicates individual subjects. To the right, the box plot central rectangle spans the first quartile to the third quartile (interquartile range [IQR]), and the horizontal line within the rectangle marks the median (the median in the no corticosteroid group is at 0 coinciding with the 25th percentile). The square symbol marks the mean. The “whiskers” above and below the box are drawn to the furthest point within 1.5 × IQR from the box (the nonoutlier range).
In this analysis of 61 mechanically ventilated SARS-CoV-2-related ARDS patients, we found that corticosteroid use was significantly associated with higher ventilator-free days at day 28; however, no significant difference was noted in hospital-free days at day 30, and in ICU-free days at day 30. To our knowledge, this is the first report that evaluated the use of corticosteroids in coronavirus disease 2019 (COVID-19) patients requiring mechanical ventilation due to ARDS.
Previously published studies drew conclusion on corticosteroids from a diverse COVID population with different diseases burdens. There is only one study to date, reporting the effect of corticosteroids on outcome in patients with SARS-CoV-2-related ARDS. In this study, Wu et al (8) reported improved survival in patients with SARS-CoV-2-related ARDS who received methylprednisolone as compared with who did not (23/50 [46%] vs 21/34 [62%]; p = 0.03). Patient who received methylprednisolone were sicker, despite of this baseline difference, there was significant mortality benefit.
In a retrospective analysis from Wuhan focused on critically ill patients with SARS-CoV-2, Yang et al (2) reported higher use of corticosteroids among survivors (14/20; 70%) as compared with nonsurvivors (16/32; 50%). Another retrospective study from Wuhan, among hospitalized patients with SARS-CoV-2 infection, there was less common use of corticosteroids among survivors (31/137; 31%) as compared with nonsurvivors (26/52; 48%) (9). However, these studies were not designed to analyze the effect of corticosteroids on specific clinical outcomes.
Our analysis has several limitations. First, this is a retrospective observational study that inferentially evaluate corticosteroids-related outcomes. Although the decision to administer corticosteroid was based on clinician preference, there was no predefined criteria to make patients eligible for that treatment. However, there was no significant difference observed in baseline characteristics and severity score in both groups, which indicates that the selection is unlikely to be biased by clinical factors. Second, this is a single center experience with relatively small sample size, which was not powered to determine mortality as a main outcome. Third, this analysis was only limited to the mechanically ventilated SARS-CoV-2-related ARDS patients, and therefore our findings cannot be generalized to all SARS-CoV-2 patients.
In summary, our analysis showed that the corticosteroids administration was associated with significant improvement in ventilator-free days at day 28, but no significant improvement in hospital- and ICU-free days at day 30 in mechanically ventilated SARS-CoV-2-related ARDS.
ACKNOWLEDGMENTS
The authors would like to thank Jeanette Mullins, BS, for her work on the data collection tool.
Footnotes
Dr. Feustel received funding as a statistical consultant forTransonic Systems, Scientific advisor with shares in Penrose TherapeuTx, LLC. Dr. Jaitovich received funding from National Institutes of Health under the award number K01-HL130704 and by the Collins Family Foundation Endowment. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Dr. Chopra is the guarantor of the article and takes responsibility for the integrity of the work, from inception to published article. Dr. Feustel was involved in performed statistical analysis. Drs. Chieng, Austin, and Jain helped with the data collection. All authors contributed to the writing of the article.
REFERENCES
- 1.Johns Hopkins University. 2020.
- 2.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med 2020; 8:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020; 323:1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323:2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell CD, Millar JE, Baillie J. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020; 395:473–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization: Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease Is Suspected. 2020. Available at: https://apps.who.int/iris/handle/10665/331446. Accessed April 12, 2020
- 7.Poston JT, Patel BK, Davis A. Management of critically ill adults with COVID-19. JAMA 2020; 323:1839. [DOI] [PubMed] [Google Scholar]
- 8.Wu C, Chen X, Cei Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 58:713–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]


