FIGURE 4.
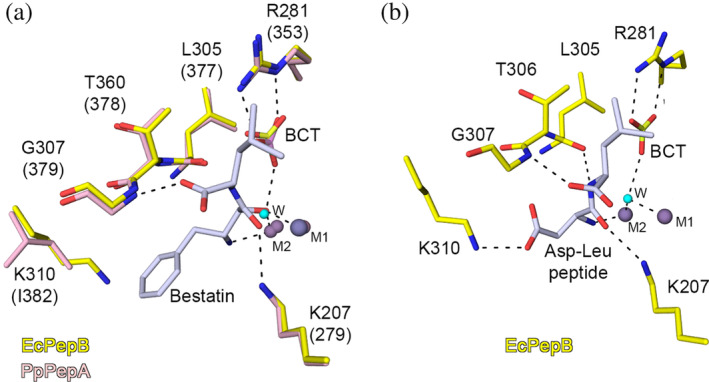
Interaction of substrate with PepB. (a) Superposition of the active sites of the Zn‐form of PepB from E. coli (PDB code 6oad, this study) and the Zn‐ and Mn‐form of the bestatin complex structure of the PepA from P. putida 6 (PDB code 3h8g). (b) Molecular modeling of the binding mode for the Asp‐Leu dipeptide in the binding pocket of the Zn‐form of E. coli PepB. Zn2+ (violet), Mn2+ (gray), and water (cyan). Carbons are colored for E. coli PepB (yellow), P. putida PepB (pink), and bestatin and Asp‐Leu dipeptide (light gray). Residue labeling is based on the sequence of PepB from E. coli and corresponding residues for P. putida are in parenthesis. Hydrogen bond interactions are shown as black dash lines. LAP, leucine aminopeptidase; PepA, aminopeptidase A; PepB, aminopeptidase B
