FIGURE 3.
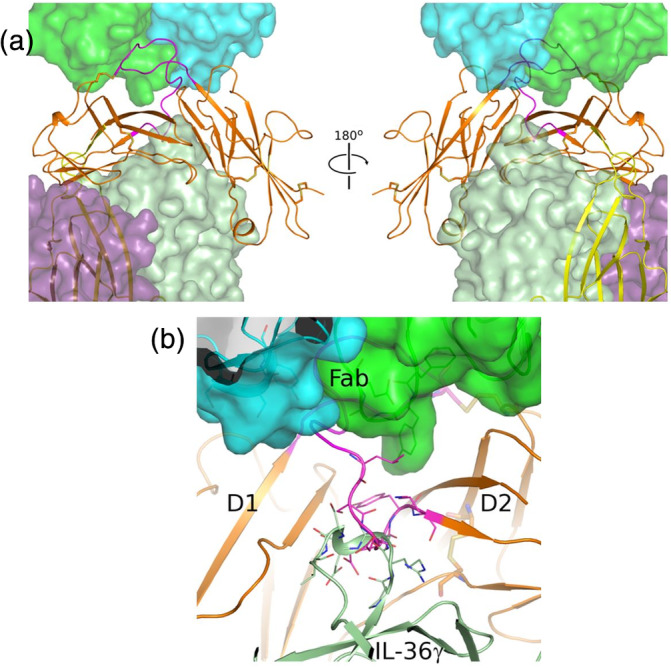
BI 655130 may act noncompetitively against IL‐36 ligand and accessory protein binding to IL‐36R. (a) Model of the quaternary complex of BI 655130 Fab:IL‐36R:IL‐36γ:IL‐36RAcP suggests that it is possible for the antagonist antibody to engage IL‐36R even when it is present as a complete signaling complex with cytokine and accessory protein. IL‐36R (D1D2) is shown as an orange cartoon and the Fab of BI 655130 is shown as a semitransparent green and cyan surface. D3 (yellow cartoon) and accessory protein (purple semitransparent surface) were placed by superposition of the IL‐1R D1D2 module from the trimeric IL‐1R:IL‐1β:IL‐1RAcP complex (PDB 4DEP 16 ) onto the D1D2 module of IL‐36R in the current structure. The bound cytokine IL‐36γ (light green semitransparent surface) was subsequently modeled by superposition of IL‐36γ (PDB 4IZE 20) onto IL‐1β in the placed IL‐1R trimeric complex. (b) Potential clash between BI 655130‐bound IL‐36R and IL‐36 ligand binding. Due to the extensive interaction between BI 655130 and the D1–D2 linker of IL‐36R, and the observed clash between the linker (magenta cartoon) and the β3–β4 loop of docked IL‐36γ (light green cartoon) in the modeled cytokine‐bound complex, an antagonist mode‐of‐action that is competitive with cytokine‐binding cannot be ruled out. This potential clash involves loops that are generally observed to be fairly mobile among IL‐receptor and cytokine structures, however, so there is uncertainty in this rigid docking pose generated by superposition and analogy with the IL‐1R signaling complex
