Figure 5.
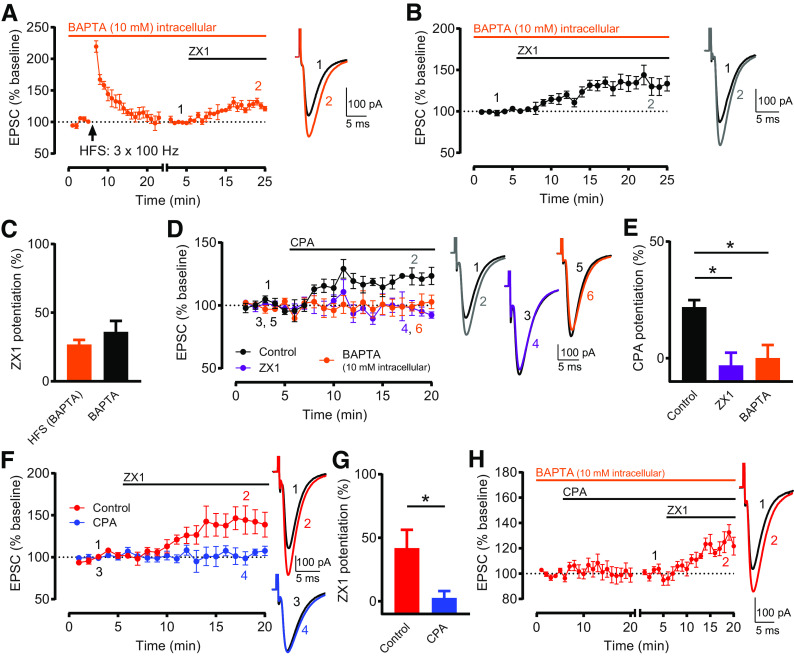
Rises in postsynaptic calcium are necessary and sufficient for inducing Z-LTD. A, Time course of AMPAR EPSC amplitude before and after HFS, and before and after subsequent ZX1 application, with intracellular recording solution containing 10 mM BAPTA. HFS did not induce LTP (n = 7, not significant, p = 0.27, paired t test). ZX1 potentiated EPSCs (n = 7, *p = 0.0019, paired t test). To examine ZX1 potentiation after HFS, similar approach and renormalization as in Figure 1C were performed. B, Time course of AMPAR EPSC amplitude before and after ZX1 application, normalized to baseline before ZX1 application, with intracellular recording solution containing 10 mM BAPTA. ZX1 potentiated EPSCs (n = 7, *p = 0.0036, paired t test). A, B, Example traces represent AMPAR EPSCs before and after ZX1. C, Average ZX1 potentiation (% increase from baseline) during the last 5 min of ZX1 application (minutes 21-25). HFS (BAPTA) (n = 7) versus BAPTA (n = 7): not significant, p = 0.3106, unpaired t test. D, Time course of AMPAR EPSC amplitude before and after CPA application (20 μM), normalized to baseline before CPA application, in controls (black), in the presence of ZX1 (purple), and with BAPTA-containing intracellular recording solution (orange). In controls, CPA potentiated EPSCs (n = 5, *p = 0.004, paired t test). In the presence of ZX1, CPA did not potentiate EPSCs (n = 4, not significant, p = 0.40, paired t test). With intracellular BAPTA, CPA did not potentiate EPSCs (n = 6, not significant, p = 0.81, paired t test). Example traces represent AMPAR EPSCs before and after CPA application. E, Average CPA potentiation (% increase from baseline) during the last 5 min of CPA application (minutes 16-20). Compared with controls (n = 5), CPA potentiation was reduced in the presence of ZX1 (n = 4, *p = 0.011), and with intracellular BAPTA (n = 6, *p = 0.013). One-way ANOVA/Bonferroni. F, Time course of AMPAR EPSC amplitude before and after ZX1 application, normalized to baseline before ZX1 application, in the presence (and prior incubation) of CPA (blue) and in controls (without CPA, red). In controls, ZX1 potentiated EPSCs (n = 5, *p = 0.036, paired t test). In CPA, ZX1 did not potentiate EPSCs (n = 5, not significant, p = 0.52, paired t test). Example traces represent AMPAR EPSCs before and after ZX1 application. G, Average ZX1 potentiation (% increase from baseline) during the last 5 min of ZX1 application (minutes 16-20). Control (n = 5) versus CPA (n = 5): *p = 0.034, unpaired t test. H, Time course of AMPAR EPSC amplitude before and after CPA application, and before and after subsequent ZX1 application, with BAPTA-containing intracellular recording solution. After obtaining a stable baseline, CPA was applied. To examine ZX1 potentiation after CPA, after obtaining a stable baseline after CPA, AMPAR EPSC amplitude was renormalized to the new baseline before ZX1 application. The renormalization is indicated by a gap and restart of timing in the x axis. With intracellular BAPTA, after CPA application, ZX1 potentiated EPSCs (n = 5, *p = 0.0016, paired t test). Example traces represent AMPAR EPSCs before and after ZX1 application.