Fig. 2. Oxidative stress–induced MTK1 activation requires both oxidation and reduction of MTK1.
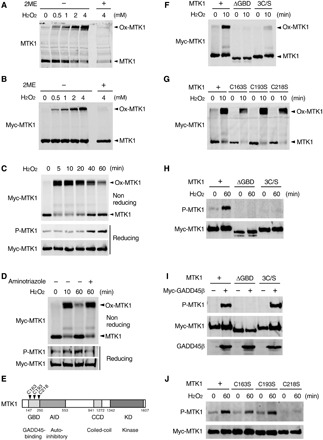
(A and B) HEK293 (A) and M57 cells (B) were stimulated with various concentrations of H2O2 for 10 min. Cell extracts were electrophoresed under nonreducing (−2ME) or reducing (+2ME) conditions, and probed for endogenous MTK1 (A) and Myc-MTK1 (B) by immunoblotting. Ox-MTK1, oxidized MTK1. (C) M57 cells were treated with H2O2 (1 mM). Cell extracts were electrophoresed under nonreducing conditions and immunoblotted with anti-Myc antibody (top). Immunoprecipitated Myc-MTK1 was electrophoresed under reducing conditions and probed with anti–P-MTK1 or anti-Myc antibodies (middle and bottom). (D) M57 cells were pretreated with the catalase inhibitor aminotriazol and stimulated with H2O2 (0.5 mM). Oxidation (top), phosphorylation (middle), and the expression level (bottom) of Myc-MTK1 were assessed as in (C). (E) Schematic diagram of MTK1 structure. GBD, GADD45-binding domain; AID, autoinhibitory domain; CCD, coiled-coil domain; KD, kinase domain. (F to H and J) HEK293 cells expressing Myc-MTK1 or its mutant derivatives (ΔGBD, 3C/S, C163S, C193S, or C218S) were treated with H2O2. Oxidation (F and G) and phosphorylation (H and J) of Myc-MTK1 were monitored as in (C). (I) Cos7 cells were cotransfected with Myc-MTK1 (WT, ΔGBD, or 3C/S) and GADD45β. Immunoprecipitated Myc-MTK1 was probed with anti–P-MTK1 or anti-Myc antibodies.
