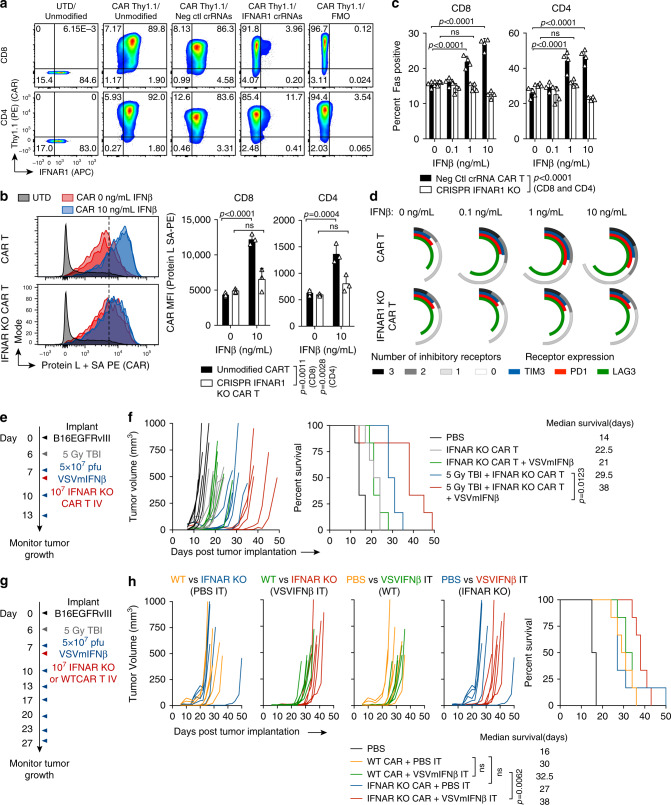
Fig. 6. Type I IFN resistant CAR T cells provide enhanced therapy with VSVmIFNβ in lymphodepleted mice.
a CAR T cells were genetically modified using CRISPR Cas9 one day after transduction by nucleofection of an RNP complex consisting of Cas9 duplexed with tracrRNA and two IFNAR1 specific or two negative control crRNAs. 48 h following modification, expression of the CAR (Thy1.1) and the IFNAR1 is shown. b Two days after modification, CAR T cells were cultured in IL2 (50 U/mL) in the absence or presence of additional recombinant mouse IFNβ. CAR expression is shown for representative CD8 CAR T cells (left) and quantified in three replicates in CD8 and CD4 CAR T cells (right). c The percent of CRISPR IFNAR1 KO or control CD8 and CD4 CAR T cells expressing Fas is shown. d Inhibitory receptor expression (PD1, LAG3, TIM3) quantified on CRISPR IFNAR1 KO or control CD8 CAR T cells cultured in IL2 in the absence or presence of additional IFNβ. Data shown are representative of two independent experiments. Technical replicates are shown ± SD (n = 3 (b) n = 4 (c)). e Mice bearing B16EGFRvIII tumors were treated with 5 × 107 pfu VSVmIFNβ or PBS 6 h prior to administration of 1 × 107 IFNAR1 KO EGFRvIII CAR T cells on day 7. Select groups received two additional doses of 5 × 107 pfu VSVmIFNβ on days 10 and 13. Select groups received a lymphodepleting dose of radiation (5 Gy TBI) on day 6. n = 6/group. f Tumor growth is shown in the left panel and overall survival is shown in right panel. g Mice bearing B16EGFRvIII tumors received a lymphodepleting dose of radiation (5 Gy TBI) on day 6 and were treated with 5 × 107 pfu VSVmIFNβ or PBS 6 h prior to administration of 1 × 107 WT or IFNAR1 KO EGFRvIII CAR T cells on day 7. Select groups received six additional doses of 5 × 107 pfu VSVmIFNβ on days 10,13,17,20,23, and 27. n = 6/group. Experiments in (e) and (g) were performed once. P-values were determined using the Log-rank Mantel-Cox test (f, h) and a two-way ANOVA with a Tukey multiple comparisons post-test (b, c). Statistical significance set at p < 0.05, ns > 0.05. Source data are provided in the Source Data File.