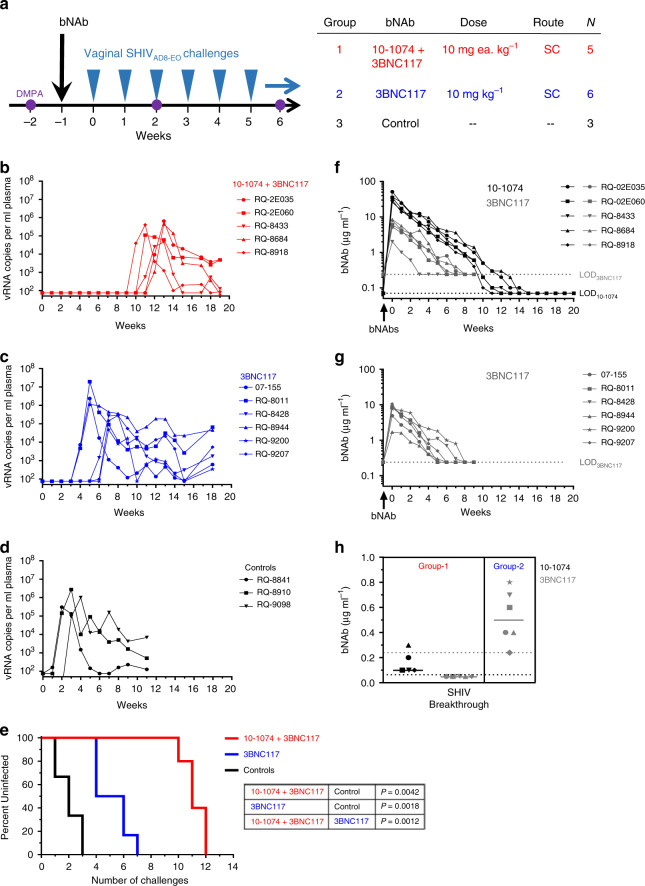
Fig. 3. Passive immunization with bNAbs 10–1074 and 3BNC117 in combination, or 3BNC117 singly, delays SHIV acquisition following repeated vaginal challenges in DMPA-treated macaques.
a Rhesus macaques (Chinese origin) were injected subcutaneously once with a combination of bNAbs 10–1074 and 3BNC117 (10 mg each kg−1; N = 5) or 3BNC117 singly (10 mg kg−1; N = 6). Commencing 1 week later, macaques were challenged repeatedly, once per week, intravaginally with SHIVAD8-EO (300 TCID50) until systemic SHIV infection was confirmed via plasma viral load assay. Control macaques (N = 3) received no antibody but were challenged identically. Animals in both groups received DMPA (30 mg) intramuscularly at 2 weeks before the first SHIV challenge and every 4 weeks thereafter to normalize SHIV susceptibility. Plasma viral loads (vRNA copies ml−1) for macaques in the combination bNAb group (b), single bNAb group (c) or untreated control group (d). Symbols denote individual animals. e Percentages of macaques remaining uninfected at 7 days following administration of the indicated cumulative number of SHIV challenges. Statistical differences between groups were analyzed using a two-sided log-rank test. Plasma levels of 10–1074 (μg ml−1) or 3BNC117 (μg ml−1) were determined via TZM-bl neutralization assays using 10–1074-sensitive pseudovirus X2088.c9 or 3BNC117-sensitive pseudovirus Q769.d22 for macaques that received both 10–1074 and 3BNC117 (f) or 3BNC117 alone (g). h Plasma concentrations of 10–1074 or 3BNC117 in individual macaques (N = 5 in Group-1, N = 6 in Group-2) at the time of SHIV breakthrough (values from f (Group-1) or g (Group-2)). Solid lines denote group medians; dotted lines indicate the limits of detection for 10–1074 (black) or 3BNC117 (gray). Source data are provided in Supplementary Tables or a Source data file.