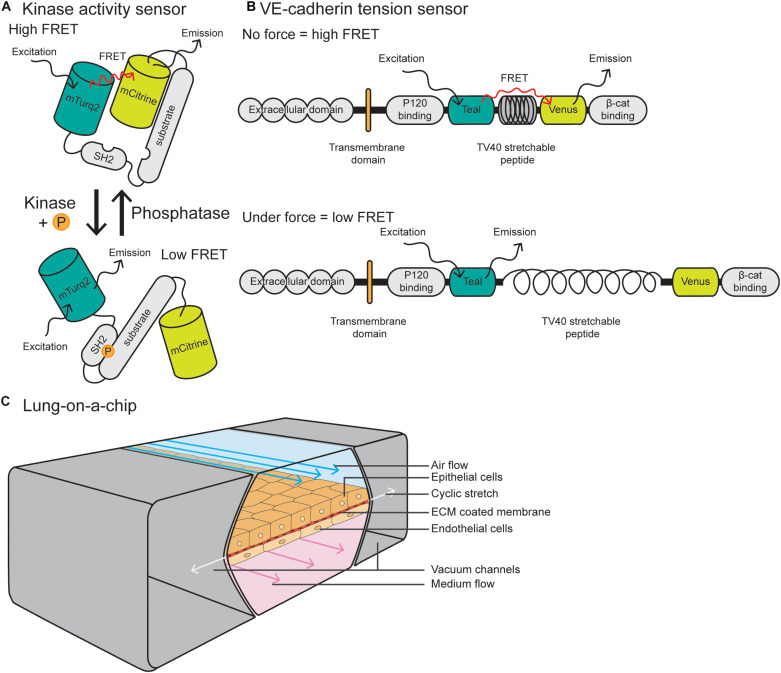
FIGURE 3.
Integrative and novel approaches to study EC forces. (A) Schematic of a Src kinase activity FRET biosensor containing mTurqoise2 as donor and mCitrine as acceptor. Upon kinase activation, the substrate becomes phosphorylated and induces intramolecular binding of the SH2 domain, resulting in a conformational change of the molecule and reduction of FRET efficiency. (B) The VE-cadherin FRET tension sensor consists of the VE-cadherin protein and the sensor module, containing Teal as donor and Venus as acceptor, connected by the TV40 stretchable peptide. The FRET sensor module was placed in between the p120-catenin and β-catenin binding sites. When there is no force applied on VE-cadherin, there is a high FRET efficiency, while force on the p120-catenin and β-catenin binding sites stretches the TV40 linker and spatially separates the Venus acceptor and Teal donor, resulting in lower FRET efficiency. (C) Schematic of a lung-on-a-chip model in which alveolar epithelial cells are plated on the apical side of an ECM-coated porous membrane, and EC are plated on the basal side. The epithelial cells are exposed to air flow and ECs are exposed to fluid flow. Cyclic stretch is applied by a vacuum in the two flanking chambers, resulting in extension and retraction of the epithelial/endothelial cell layer in the center.