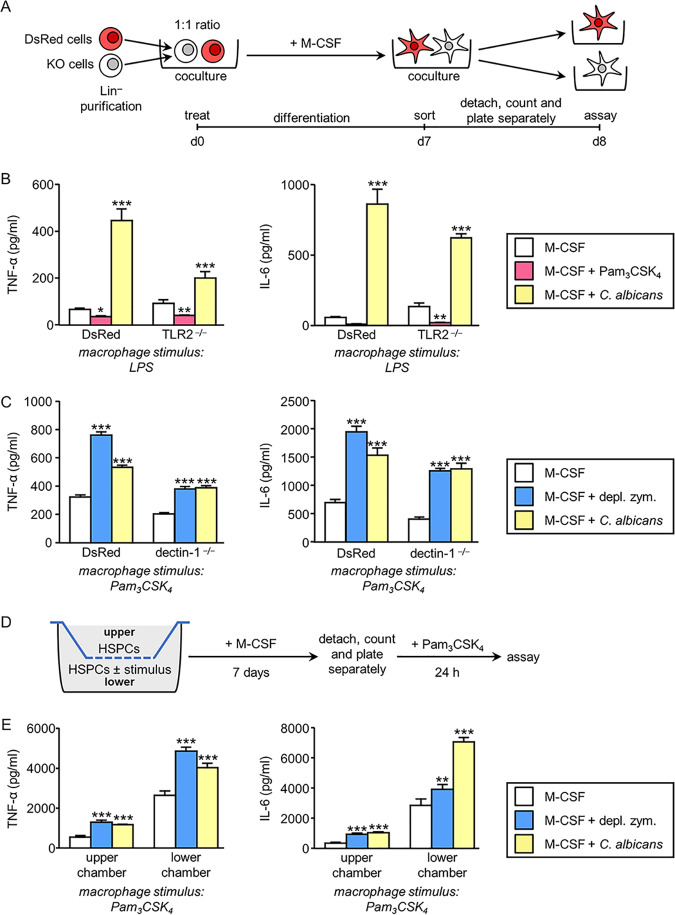
FIG 5.
Responsiveness of macrophages derived from Lin− cells differentiated in vitro in response to M-CSF ± stimulus. (A) Schematic protocol of in vitro Lin− cell differentiation and sorting (see Materials and Methods). DsRed and TLR2−/− Lin− cells (B) or DsRed and dectin-1−/− Lin− cells (C) were cultured at a 1:1 ratio and stimulated with M-CSF in the absence or presence of inactivated yeasts of C. albicans or Pam3CSK4 (B) or depleted zymosan (C) for 7 days. Cells were labeled with anti-CD11b and anti-F4/80 antibodies, and DsRed-positive and -negative macrophages (CD11b+ F4/80+) were sorted by flow cytometry and plated separately. (B and C) Macrophages were stimulated with LPS (100 ng/ml) or Pam3CSK4 (100 ng/ml), and TNF-α and IL-6 levels in 24-h culture supernatants were assessed by ELISA. Triplicate samples were analyzed in each assay. Results are expressed as means ± SD of pooled data from three experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 with respect to cytokine production by macrophages derived from Lin− cells differentiated with M-CSF only, in the absence of additional stimuli. (D) Schematic protocol of in vitro Lin− cell differentiation in transwell assays (see Materials and Methods). (E) Macrophages from the upper or lower chamber were separately stimulated with Pam3CSK4 (100 ng/ml), and TNF-α and IL-6 levels in 24-h culture supernatants were assessed by ELISA. Triplicate samples were analyzed in each assay. Results are expressed as means ± SDs of pooled data from three experiments. **, P < 0.01; ***, P < 0.001 with respect to cytokine production by macrophages derived from Lin− cells differentiated with M-CSF, only in the absence of additional stimuli in the lower chamber.