Table 2. Standard Gibbs Free Energy Change (ΔGf°) and Formation Constant (Kf, log Kf) for the Chelation of Cu(II) with AG (as per eq 11) in Aqueous Solution at 298.15 Ka.
| complex [Cu(AG)x(H2O)n]2+ |
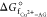 (kcal/mol) (kcal/mol) |
||||
|---|---|---|---|---|---|
| [5] [Cu(AGA)(H2O)2]2+ (N1, N2) | –4.0 | 8.84 × 102 | 2.95 | ||
| [6] [Cu(AGA)(H2O)3]2+ (N2) | –11.7 | 3.64 × 108 | 8.56 | ||
| [7] [Cu(AGB)(H2O)2]2+ (N1, N4) | –3.9 | 6.95 × 102 | 2.84 | ||
| [8] [Cu(AGB)(H2O)3]2+ (N2) | –10.6 | 5.76 × 107 | 7.76 | ||
| [9] [Cu(AGC)(H2O)2]2+ (N1, N4) | –4.1 | 9.91 × 102 | 3.00 | ||
| [10] [Cu(AGC)(H2O)3]2+ (N2) | –11.7 | 3.48 × 108 | 8.54 | ||
| [11] [Cu(AGD)(H2O)2]2+ (N2, N4) | –21.9 | 1.10 × 1016 | 16.04 | ||
| [12] [Cu(AGA)2]2+ (N1, N2) | –7.4 | 2.65 × 105 | 5.42 | ||
| [13] [Cu(AGA)2]2+ (mirror im., N1, N2) | –7.1 | 1.48 × 105 | 5.17 | ||
| [14] [Cu(AGA)2(H2O)2]2+ (N2) | –24.8 | 1.47 × 1018 | 18.17 | ||
| [15] [Cu(AGB)2]2+ (N1, N4) | –7.6 | 3.53 × 105 | 5.55 | ||
| [16] [Cu(AGB)2]2+ (mirror im., N1, N4) | –7.3 | 2.35 × 105 | 5.37 | ||
| [17] [Cu(AGB)2(H2O)]2+ (N1, N4, N2′) | –15.8 | 3.98 × 1011 | 11.60 | ||
| [18] [Cu(AGB)2(H2O)2]2+ (N2) | –24.1 | 4.82 × 1017 | 17.68 | ||
| [19] [Cu(AGC)2]2+ (N1, N4) | –7.7 | 4.40 × 105 | 5.64 | ||
| [20] [Cu(AGC)2]2+ (mirror im., N1, N4) | –7.5 | 3.12 × 105 | 5.49 | ||
| [21] [Cu(AGC)2(H2O)]2+ (N1, N4, N2′) | –16.1 | 6.70 × 1011 | 11.83 | ||
| [22] [Cu(AGC)2(H2O)2]2+ (N2) | –24.5 | 8.52 × 1017 | 17.93 | ||
| [23] [Cu(AGD)2]2+ (N2, N4) | –40.8 | 8.24 × 1029 | 29.92 | ||
| [24] [Cu(AGD)2]2+ (mirror im., N2, N4) | –40.9 | 9.91 × 1029 | 30.00 | ||
| [25] [Cu(AGD)2(H2O)]2+ (N2) | –31.0 | 4.87 × 1022 | 22.69 | ||
| [26] [Cu(AGD)2(H2O)2]2+ (N2) | –22.1 | 1.52 × 1016 | 16.18 | ||
| [27] [Cu(AGA)(AGD)]2+ (N1, N2, N2′, N4) | –25.1 | 2.48 × 1018 | 18.39 | ||
| [28] [Cu(AGB)(AGD)]2+ (N1, N4, N2′, N4) | –24.2 | 5.28 × 1017 | 17.72 | ||
| [29] [Cu(AGC)(AGD)]2+ (N1, N4, N2′, N4) | –25.8 | 8.86 × 1018 | 18.95 | ||
| [30] [Cu(AGC)(AGD)]2+ (mirror im., N1, N4, N2′, N4) | –24.4 | 7.58 × 1017 | 17.88 |
Coordinating atoms in the organic ligand are shown in parentheses for each complex; when two AGs are in different conformations or have different coordinating atoms, these are distinguished using a prime (′).
