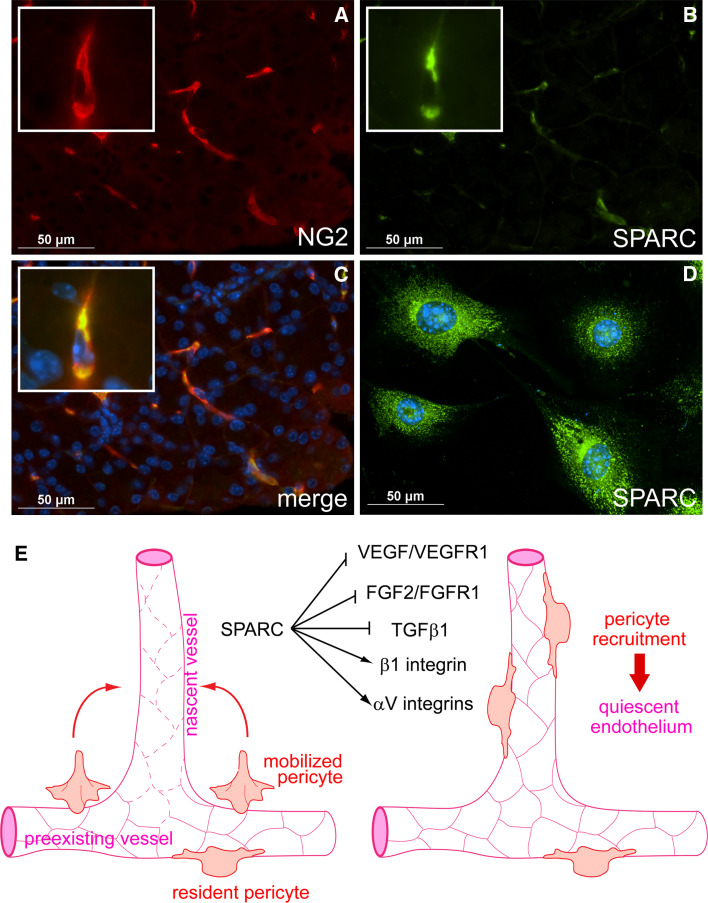
Fig. 1.
Regulation of angiogenesis by SPARC. a–c Pericyte expression of SPARC in murine pancreas. Pancreas was harvested from a 3-week-old mouse, sectioned, and subjected to immunofluorescent staining for the pericyte marker NG2 (a) and SPARC (b). The merged image is presented with nuclear DAPI staining in c. Pericytes express SPARC in vitro (d). Whole murine pancreata were subjected to collagenase digest; NG2+ cells were then isolated using anti-NG2 IgG immunomagnetic bead separation. Early passage cells were subjected to immunofluorescent staining for SPARC. SPARC staining is presented with nuclear DAPI staining in d. Note cytoplasmic staining of SPARC in b and d. e Model illustrating potential regulation by SPARC of integrin activity and multiple growth factor signaling pathways critical to angiogenesis. During angiogenesis, VEGF, FGF2, and TGF-β1 are secreted by cells or released from the ECM into an angiogenic milieu. These growth factors induce formation of new blood vessels that are marked by excessive leakiness and endothelial cell proliferation (left diagram; leaky vessels are indicated by dashed endothelial cell–cell junctions). Pericytes are mobilized and secrete SPARC into the angiogenic microenvironment, where it negatively regulates VEGF/VEGFR1 and FGF2/FGFR1 signaling, diminishing endothelial cell proliferation. SPARC also influences the activities of β1, αVβ3, and αVβ5 integrins. SPARC binds β1 integrin and stimulates downstream signaling in an interaction that may adversely affect ECM interactions. In contrast, SPARC seems to promote αV integrin–ECM interactions. SPARC also regulates activation of TGF-β1 signaling, however, the mechanism and outcome of this regulation is unclear and is perhaps cell-type-dependent. Evidence in support of an inhibitory activity of SPARC that blocks the activation of TGF-β1 in pericytes has been reported [14, 37, 40]. SPARC inhibition of TGF-β1 gives pericytes opportunity to migrate to the nascent vessel and engage the activated endothelium (right diagram). Subsequently, TGF-β1 is activated and pericytes induce vessel quiescence. The resulting mature vessel thus consists of both pericytes and endothelial cells. In total, the diversity of pathways that are influenced by SPARC places it as an important regulator of microenvironment-perception