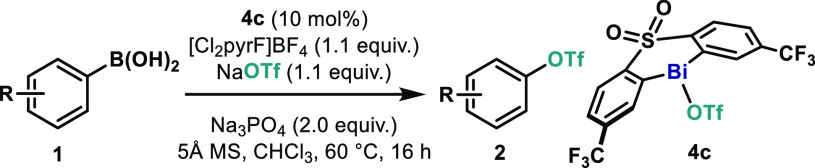
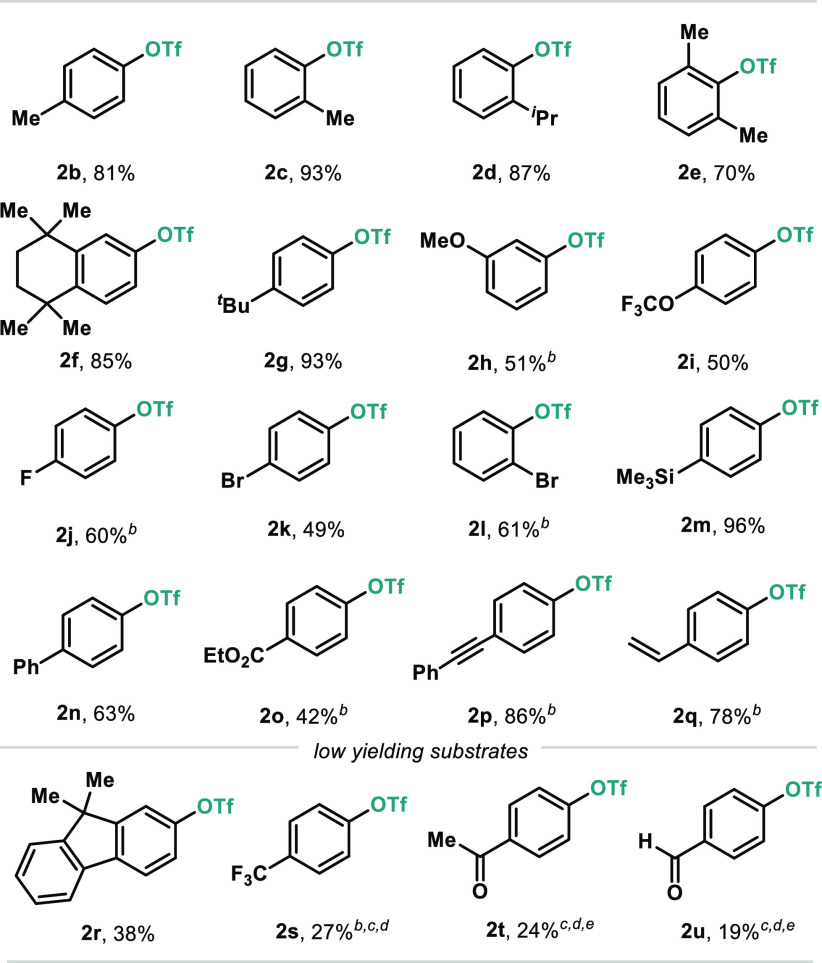
Table 2. Scope of the Bi-Catalyzed Oxidative Coupling of Arylboronic Acids and Sodium Triflatea.
Reaction conditions: 1 (0.3 mmol), NaOTf (0.33 mmol), 4c (0.03 mmol), [Cl2pyrF]BF4 (0.33 mmol), Na3PO4 (0.6 mmol), and 5 Å MS (120 mg) in CHCl3 at 60 °C for 16 h. Yields of isolated pure material.
Reaction performed at 90 °C with 2.0 equiv of NaF as base.
Yields determined by 19F NMR using 1-fluoro-4-nitrobenzene as internal standard.
Reactions performed at 0.025 mmol of the corresponding arylboronic acids.
Reaction performed at 90 °C with 4.0 equiv of Na3PO4 as base.