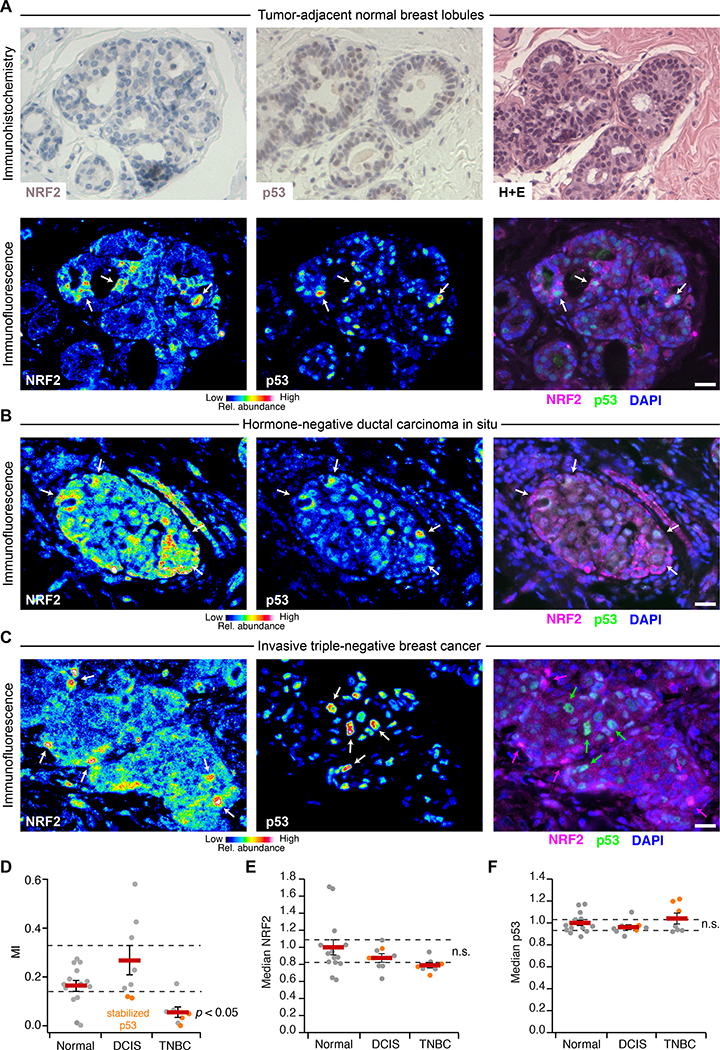
Fig. 6. NRF2 and p53 are co-stabilized in breast epithelial tissue and premalignant lesions but uncoupled in TNBC.
(A) Immunohistochemistry (upper) and immunofluorescence (lower) for NRF2 and p53 in tumor-adjacent normal breast lobules. Hematoxylin and eosin (H+E, upper right) histology is from a serial paraffin section for p53. Images from a tumor-adjacent normal breast duct are shown in fig. S17. (B and C) Multicolor immunofluorescence for NRF2 and p53 in (B) hormone-negative ductal carcinoma in situ and (C) triple-negative breast cancer. (D) Quantification of the association between NRF2 and p53 immunoreactivities represented in (A) to (C). (E and F) Median NRF2 and p53 immunoreactivities for the designated tissue type in each clinical case. n.s., not significant (p > 0.05). For (A) to (C), immunofluorescence is shown as representative pseudocolored images for NRF2 (left) and p53 (middle) are shown merged with DAPI nuclear counterstain (right). White arrows indicate concurrent NRF2 and p53 stabilization, and magenta or green arrows indicate stabilization of NRF2 or p53 separately. Scale bars are 20 μm. For (D) to (F) data are mean ± s.e.m. of n = 14 cases with tumor-adjacent normal epithelium (Normal), 8 cases with ductal carcinoma in situ (DCIS), and 7 cases of triple-negative breast cancer (TNBC). Multi-group comparison was made by Kruskal-Wallis rank-sum test with Šidák correction for multiple-hypothesis testing.