Figure 1.
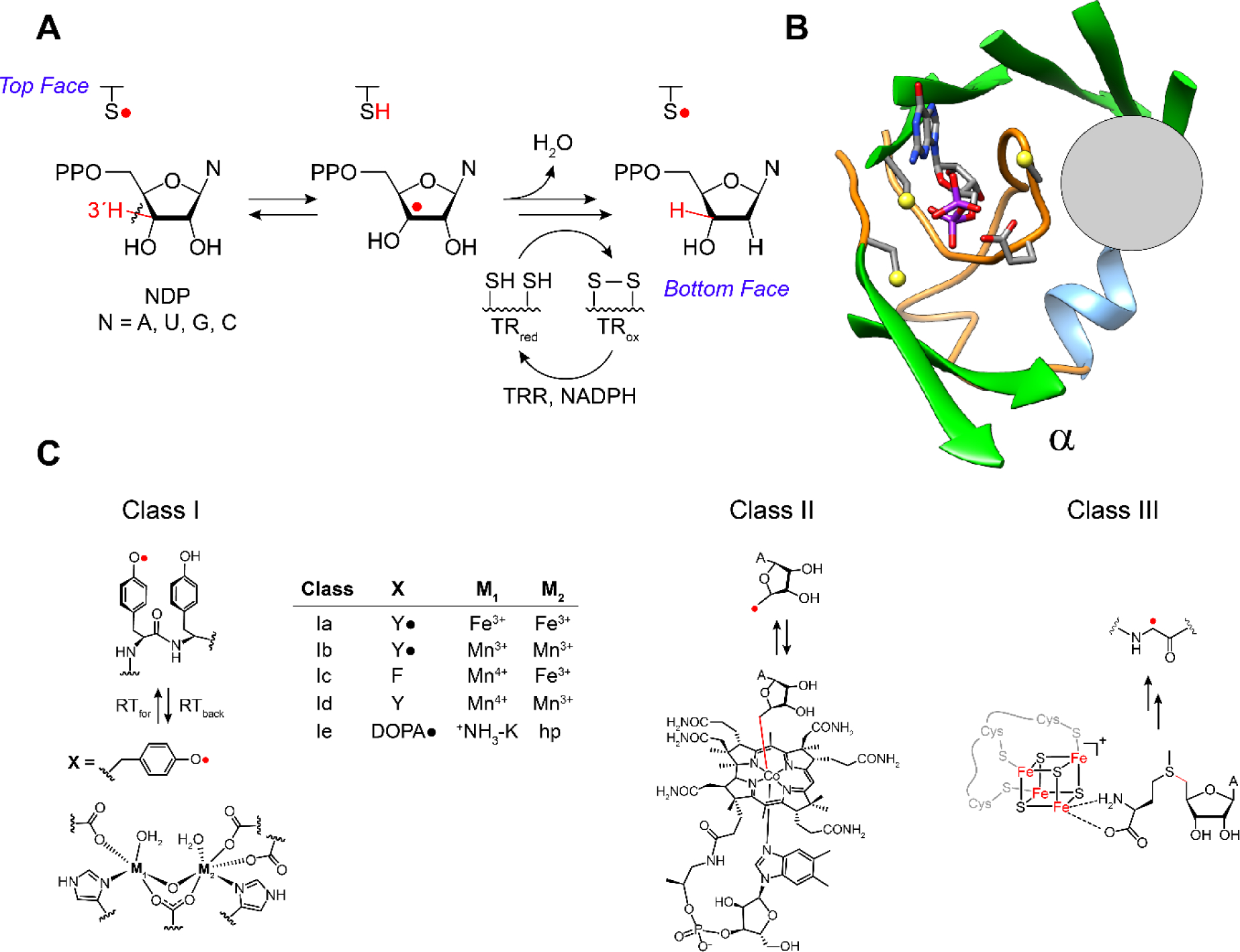
A RNRs catalyze the conversion of nucleoside di- or triphosphates, ND(T)Ps, to deoxynucleoside di- or triphosphate, dND(T)Ps. B The reduction occurs in the active site in subunit α composed of a 10 stranded β barrel with three cysteines and the conserved placement of the oxidant (gray circle, panel B) involved in thiyl radical formation (−S•, top face in A) that initiates NDP reduction. The bottom face thiols in A deliver the reducing equivalents and themselves become oxidized. C The oxidants are distinct among the RNR classes (I, II and III) represented here by a gray circle that is juxtaposed with the thiyl radical loop. Substrate and four essential residues, including the three essential cysteines and E441, are shown as sticks. C The class Ia RNRs use a diferric-tyrosyl radical (Y•) cofactor (M1, M2 = Fe3+) that is located in subunit β (left, bottom) to regenerate a radical species in the active site in subunit α. The oxidation occurs over a distance of ~33 Å by long range radical transfer to first generate a Y• in subunit α (under the gray circle), and second generate -S• on an adjacent cysteine (top face in A). In other class I RNRs (Ib-Ie) the oxidation also occurs by long range radical transfer across α and β, but involves distinct metallo-oxidants (X, M1, M2). In the case of the class II and III RNRs the oxidants, the 5′-deoxyadenosyl radical generated from adenosylcobalamin (class II) and the glycyl radical (class III) generated from S- adenosylmethionine and an FeS cluster, are located adjacent to the cysteine to be oxidized (gray circle). A = adenine base.
