Figure 7.
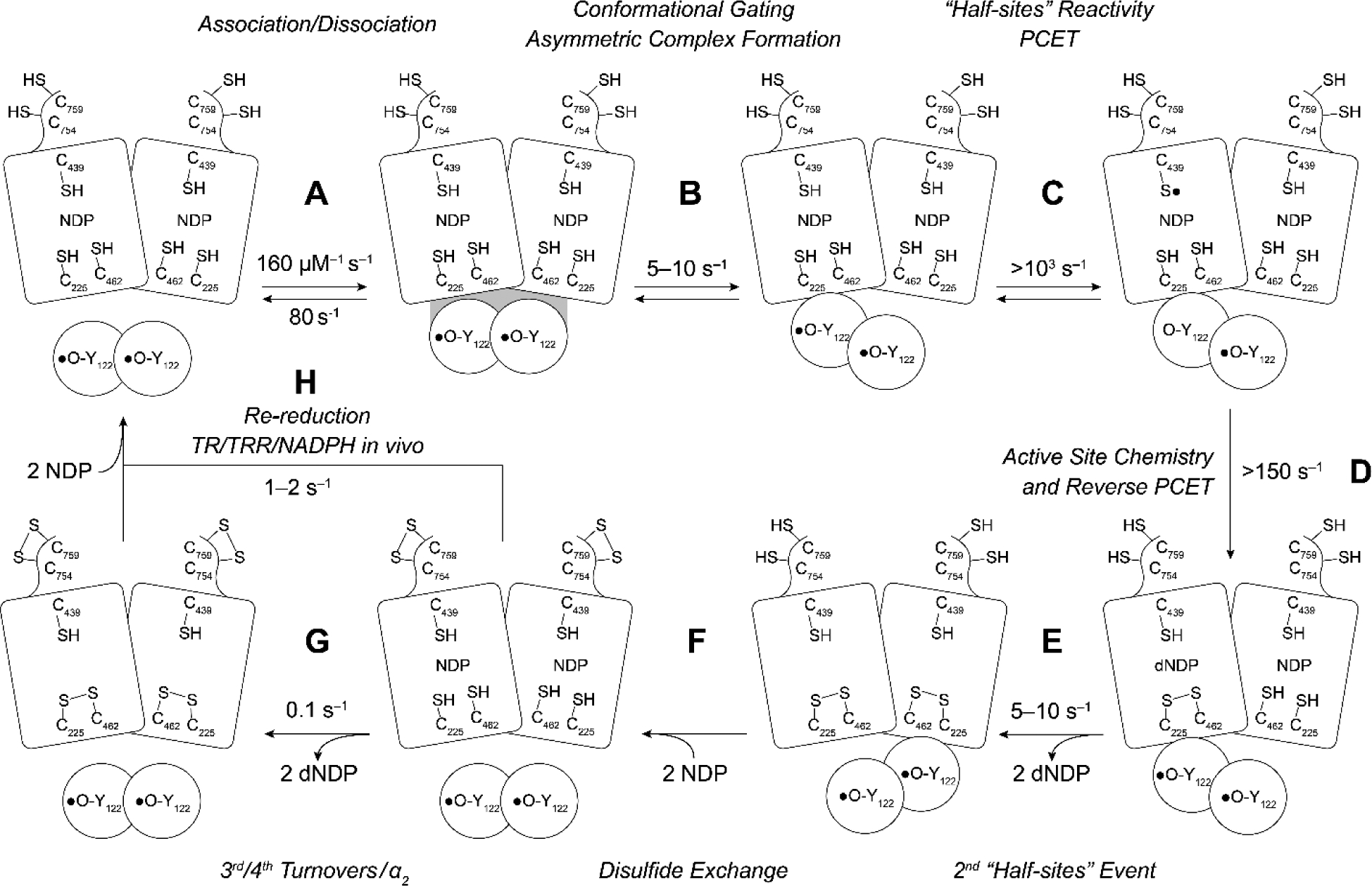
Resetting RNR for single and multiple turnovers. The model assumes a 1:1 α to β ratio, that Y122• is distributed equally between each β, and that the wt-α2β2 complex is asymmetric. In the absence of external reductants, 2 dCDPs are generated at 2 (substrate only) to 5–10 s−1 (substrate and effector) that arise from chemistry at each α of α2 (step E). Steps B and E are rate-limiting and conformationally gated. In an assay in the absence of an external reductant, two additional dCDPs are formed at 0.1 s−1 (step G). In the presence of an external reductant such as thioredoxin (TR) and TR reductase (TRR), under steady state conditions, step H becomes rate-limiting.
