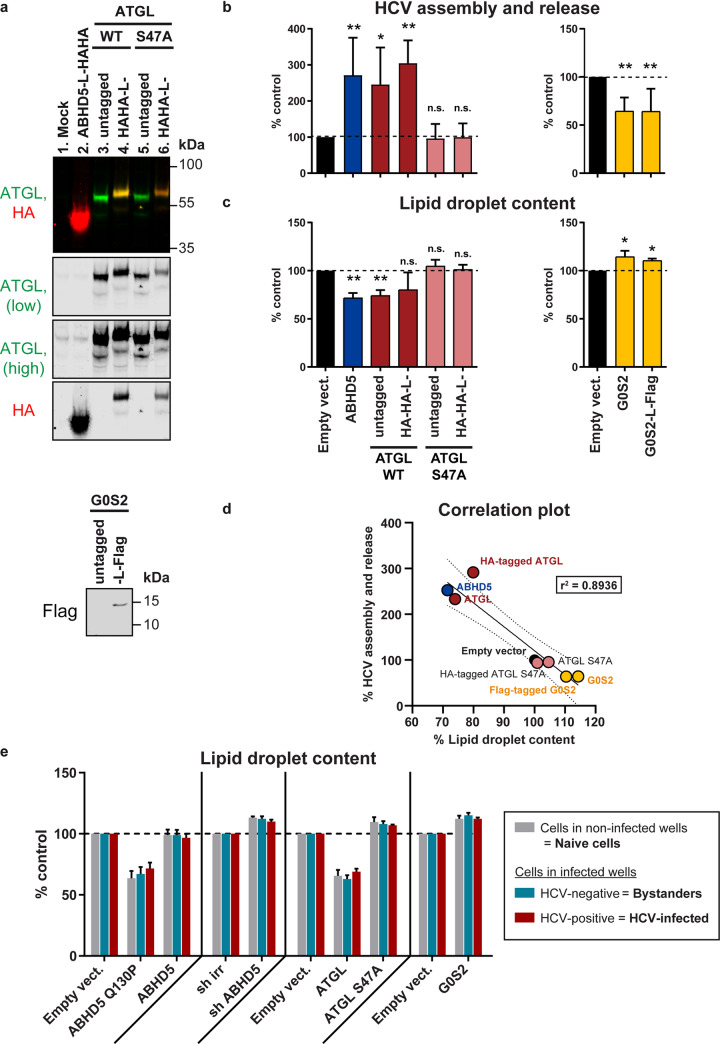
Fig 4. Endogenous or ectopically expressed ATGL controls lipid droplet lipolysis and HCV assembly.
(a) Western blot verification of ABHD5, ATGL and G0S2 expression 72 hours after lentiviral transduction in Lunet N hCD81 cells. Proteins were detected with antibodies directed against ATGL, or the HA and Flag epitopes. For ATGL detection, the endogenous protein can be seen in the high contrast picture (lanes 1 and 2). (b) Effect of ABHD5, ATGL or G0S2 expression on HCV assembly and release. HCV production was determined in a whole replication cycle assay with the JcR2a virus in Lunet N hCD81 Fluc cells and normalized for replication (n = 6 for left panel, n = 7 for right panel). (c) Effect of ABHD5, ATGL or G0S2 expression on lipid droplet lipolysis. Lipid droplet content was measured by flow cytometry 72 hours post-lentiviral transduction in Lunet N hCD81 cells (n = 4 for all conditions except HA-tagged ATGL constructs and Flag-tagged G0S2 where n = 3). (d) Correlation between HCV production and lipid droplet lipolysis. This graph gathers the results plotted in panels b and c as well as the linear regression (full line), 95% confidence band (dotted lines) and r2 value. Data in panels b, c and d were normalized to empty vector- transduced cells. (e) Effect of ABHD5, ATGL or G0S2 expression on lipid droplet lipolysis in naive, bystanders and HCV-infected cells. Lunet N hCD81 cells were lentivirally transduced with the constructs indicated on the X axis and infected 48 hours later with HCV Jc1. Two days later, the cells were harvested, mixed with mRuby2-expressing reference cells and fixed, as explained in S2A Fig. In this case however, the cells were permeabilized and stained with an antibody against HCV NS5A to determine their infection status, in addition to the lipid droplet dye BODIPY. Naive, bystanders and HCV-infected cells were gated as depicted in S3B Fig. Their lipid droplet content was measured and normalized for the reference cell population, to correct for staining or measurement variations. For each condition, the results were then normalized to the respective empty vector-transduced cell population (n = 3).