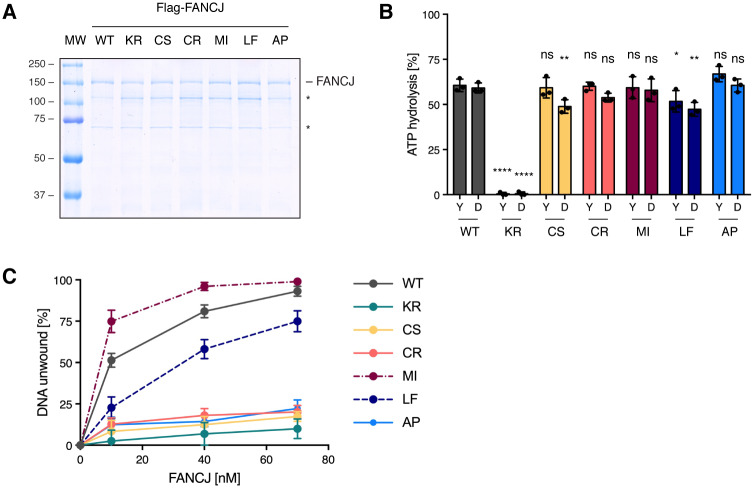
Fig 2. The FeS cluster in FANCJ is indispensable for helicase activity.
(A) InstantBlue stained SDS-PAGE gel of purified N-terminally Flag-tagged FANCJ variants. Asterisks mark contaminants. (B) ATP hydrolysis of FANCJ variants in the presence of an oligonucleotide-based Y-structure (Y) or D-loop (D) substrate, as measured by the release of inorganic phosphate from radio-labelled γ-32P-ATP in thin-layer chromatography. Activity is depicted as % of hydrolysed ATP, with background activity in the absence of DNA subtracted. Error bars depict standard deviations from three independent experiments. Statistical analysis: ordinary one-way ANOVA (****, p < 0.0001; **, p < 0.01; *, p < 0.1; ns, non-significant). (C) Graphical representation of DNA unwinding of a D-loop substrate with increasing concentrations of FANCJ variants. In the graph, mean values and standard deviations of three independent experiments are depicted. WT, wild-type; KR, K52R; CS, C283S; CR, C283R; MI, M299I; LF, L340F; AP, A349P. See also S2 Fig.