Abstract
Purpose of Review:
Throughout the lifespan, lung injury impedes the primary critical function essential for life-respiration. To repair quickly and efficiently is critical and is orchestrated by a diverse repertoire of progenitor cells and their niche. This review incorporates knowledge gained from early studies in lung epithelial morphogenesis and cell fate and explores its relevance to more recent findings of lung progenitor and stem cells in development and regeneration.
Recent Findings:
Cell fate in the lung is organized into an early specification phase and progressive differentiation phase in lung development. The advent of single cell analysis combined with lineage analysis and projections is uncovering new functional cell types in the lung providing a topographical atlas for progenitor cell lineage commitment during development, homeostasis, and regeneration.
Summary:
Lineage commitment of lung progenitor cells is spatiotemporally regulated during development. Single cell sequencing technologies have significantly advanced our understanding of the similarities and differences between developmental and regenerative cell fate trajectories. Subsequent unraveling of the molecular mechanisms underlying these cell fate decisions will be essential to manipulating progenitor cells for regeneration.
Keywords: Lung development, progenitor cells, cell fate, lung regeneration, differentiation
Introduction
The mammalian lung is an origami of epithelial and vascular tubes folded into a matrix of mesenchyme. This structure allows an organization of over 40 diverse cell types to orchestrate the fundamental functions of the lung including respiration, inflammation sensing and barrier protection, and metabolism and homeostasis (Table 1) [1]. Building and rebuilding a complex structure requires precision. A critical challenge for this is the coordination of cellular differentiation and maintenance with the patterning/morphogenesis programs that are specific and unique to lung development and regeneration.
Table 1.
Functional epithelial cells of the lung
| Location | Cell Type | Cell Markers | Function |
|---|---|---|---|
| Conducting Airway | Secretory (Club) Cells | SCGB3A1, SCGB1A1 |
|
| Multiciliated Cells | FOXJ1, TUBB4 |
|
|
| Basal Cells | KRT5, TRP63 |
|
|
| Neuroendocrine Cells | UCHL1, CALCA, ASCL1 |
|
|
| Tuft (Brush) Cells | IL25, TSLP, DCLK1 |
|
|
| Ionocytes | FOXI1, ATP6V1C2 |
|
|
| Goblet Cells | MUC5AC, TFF2, SPDEF |
|
|
| Alveolar | Type 1 Cells | HOPX, AQP5, PDPN, AGER |
|
| Type 2 Cells | SFTPC, SFTPB, SFTPA, SFTPD, ABCA3 |
|
Evolution has shaped development in multiple organ systems including the lung. The lung employs a spatiotemporal system of morphogens to not only pattern the lung but also simultaneously specify and lineage restrict progenitor cells. By deploying expression gradients, morphogens can multitask via inductive, autocrine, short-range and long-range signaling to instruct cell identity and structural morphogenesis in a coordinated fashion. This paradigm ensures a stereotypical roadmap for lung development and a guide for regeneration after lung injury.
In this review, we will summarize the essential elements of lung development. We will focus on some of the molecular mechanisms governing tissue interactions and epithelial progenitor cell fate projection during development. Finally, we will highlight how similar fetal programs may underlie the innate plasticity observed in progenitor cells in the adult lung that differ from progenitors present during development and carry out normal lung homeostatic functions.
Specification of multipotent epithelial progenitor cells during lung development
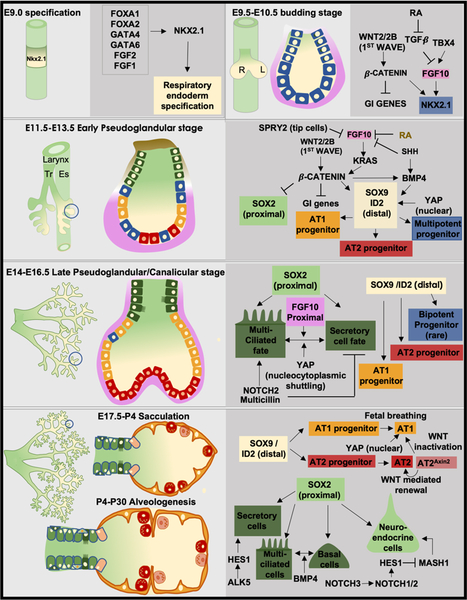
The lung undergoes a series of developmental phases that include the embryonic, pseudoglandular, canalicular, saccular, and alveologenesis stages. In the mouse, lung identity is first established during the embryonic phase of lung development when morphogens in the surrounding mesoderm induce expression of the transcription factor, NKX2–1, in the ventral anterior foregut endoderm around embryonic day 9.0 (E9.0) in the mouse. NKX2–1 expression not only assigns lung identity to the endoderm, but it is also a pioneering transcription factor required for lung development [2]. Soon after lung identity is established, outpouching of the endoderm into the surrounding mesoderm to form the primitive trachea and subsequent growth of paired lung buds occurs (Figure 1A, B).
Figure 1: Epithelial progenitor specification and differentiation during lung development.
(A) Lung identity is established at E9.0 with the induction of the transcription factor Nkx2.1 expression (yellow) by a combination of morphogens and transcription factors shown in the dark grey box (B) This is subsequently followed by the outpouching of the respiratory endoderm to form trachea and primary lung buds. The whole mount view of the lung is shown in the left while the morphology of a single branch is shown in the right side. The network of genes involved in maintaining Nkx2.1 expression (blue cells) is shown to the right. (C) By E12.5, the pseudoglandular stage involves the separation of the trachea (Tr) from the esophagus (Es). Whole mount view of the lung is on the left and the morphology of a single branch is on the right. During this stage, reciprocal mesodermal–endodermal interactions that govern the specification of Nkx2.1+ multipotent progenitor cells into a Sox2+ proximal (green cells) and Sox9+/Id2+ distal fate with concurrent alveolar cell fate specification programs (blue, red, orange cells) are shown in dark grey. (D) By the end of the pseudoglandular and into the canalicular stage, the majority of branching is complete. Whole mount view of the lung is on the left and the morphology of a single branch is on the right. The proximal Sox2+ progenitors begin to give rise differentiated cell lineages such as multiciliated and secretory cells (dark green cells). At the distal end, the specification of the Sox9+ cells to the alveolar fate is complete. The corresponding gene network involved in the specification is shown. (E) The proximal Sox2+ progenitors give rise to all proximal cell types: club cells, multi-ciliated cells, basal cells and neuroendocrine cells (green cells). The distal alveolar progenitors give rise to AT1s and AT2s (orange and red cells). The signaling networks that contribute to the differentiation of the progenitors are shown in the side.
Commencing around E12.5 during the pseudoglandular stage of development, reciprocal mesodermal-endodermal interactions govern branching morphogenesis and specification of multipotent progenitor cells into a proximal or distal fate. This sets in motion the establishment of proximal or conducting airway lineages marked by expression of SOX2 and those of the distal alveolar epithelial region expressing the transcription factors ID2 or SOX9 (Figure 1C) [3, 4].
As branching morphogenesis progresses into the canalicular stage around E15.5 of lung development, the proximal SOX2+ progenitor cells are specified and presumably differentiate into many of the epithelial cells of the conducting airway and trachea including the pulmonary neuroendocrine cells, secretory or club cells, multiciliated cells, basal cells, goblet cells, tuft cells, and newly described ionocyte cells (Table 1) [5–12]. Whether all SOX2+ progenitor cells at this stage are multipotent or exist as multiple subsets of oligopotent or even unipotent progenitor cells is unknown (Figure 1D).
Several earlier studies indicate that distal lung specification of the SOX9/ID2+ progenitor cells is a progressive process that eventually leads to differentiation into the type 1 (AT1) and type 2 (AT2) alveolar epithelial cells (Table 1) [13, 14]. In addition, recent evidence suggests that specific alveolar cell fate specification programs are initiated concurrent with branching morphogenesis in the pseudoglandular and canalicular stages [15]. Alternatively, it has been proposed that the distal tip endodermal progenitor cells evolve into a bipotent progenitor cell capable of becoming AT1s or AT2s during the saccular stage (Figure 1E) [16, 17]. However, the relative contribution of bipotent cells to mature alveolar lineages appears to be minimal, and it may represent residual, progressively differentiating cells [15]. Nevertheless, alveolar epithelial cell fate is likely represented by early specification and acquisition of cell identity via predominantly unilineage progenitors with additional contribution through bilineage progenitor cells.
As our understanding grows of how progenitor cell fate identity is temporally and spatially established, a recurring theme arises in that a common set of molecular mechanisms and morphogens regulate both morphogenesis and cell specification. Evolution has equipped the lung with efficiency. While there is contribution from an abundant number of morphogens and their downstream signaling pathways, we outline below a few major players.
Role of WNT signaling pathway in coordinating epithelial differentiation
The signaling network comprising WNT family of ligands and receptors and their downstream effectors is one of the fundamental pathways that directs cell proliferation, cell polarity, and determination of cell fate throughout embryonic development and postnatal homeostasis and regeneration [18]. WNT proteins function as morphogens that are capable of both short- and long-range signaling. They are regulated by lipidation, membrane microdomain proteins, and distribution by heparan sulfate proteoglycans to regulate the ligand concentration, diffusion, and distribution through the aqueous extracellular space.
While both canonical and non-canonical pathways of WNT signaling regulate lung morphogenesis [19], canonical WNT/β-catenin-dependent signaling plays a dominant role in airway specification during lung morphogenesis. The role of WNT in progenitor specification during the fetal and postnatal stages can be understood in the context of two temporally segregated waves of WNT signaling. The first wave of WNT signaling is concomitant with the specification of the foregut into the respiratory lineage and lasts up till mid pseudoglandular phase of lung development. WNT ligands emanating from the surrounding mesoderm signal to the anterior foregut endoderm to activate WNT signaling and specify initial lung identity [20–22]. Intriguingly, while β-catenin maintains NKX2–1 expression to repress SOX2 and gastrointestinal (GI) identity genes early after specification, its function is restricted to promotion of SOX9 expression and repression of GI genes only later in early development [21]. Later in lung development, loss of β-catenin or ectopic expression of the WNT inhibitor, DKK1, in the lung epithelium also affects airway endoderm identity with loss of distal epithelial lineages and proximalization of airways, a process acting upstream of BMP4, FGF signaling and N MYC [23, 24]. Interestingly, ectopic activation of WNT signaling in a more distal and later context is dependent on the presence or absence of endogenous TCF-LEF factors [25, 26]. Towards the end of branching morphogenesis, WNT-responsiveness in the lung diminishes with a few responsive cells confined to the SOX9+/SOX2− distal lung progenitors [27]. However, following late sacculation occurring in the early postnatal period in mice, there is a re-emergence of WNT signaling and WNT-responsive AT2 cells that promotes postnatal alveolar progenitor expansion and balances the epithelial composition of the alveolus through control of AT2-AT1 progenitor differentiation [27].
FGF signaling in the mesenchyme coordinates epithelial progenitor specification and differentiation
Fibroblast growth factor (FGF) signaling components include eighteen ligands and four receptor tyrosine kinases that propagate signaling via a vast number of downstream effectors [28]. FGF signaling is critical through all stages of life from coordinating organogenesis to tissue regeneration by regulating proliferation, specification, survival and metabolism.
Initial specification of lung endoderm relies on signals from the splanchnic mesoderm that includes the secondary heart field. Included in this group is FGF2 that is secreted from the cardiac mesoderm and has the potential to induce lung identity from foregut endoderm [29]. FGF10 is a morphogen secreted by the lung mesenchyme that plays a paramount role in the regulation of epithelial proliferation and lineage commitment during embryonic and postnatal development and regeneration post-injury [30, 31]. Lineage tracing FGF10+ cells in mice has revealed temporally segregated waves of FGF expression in the lung primordium [32]. The first wave initiates post lung specification during the early pseudoglandular stage with the FGF10+ cells localizing to distal (sub-mesothelial) mesenchyme surrounding the airways. While FGF10 plays an essential role in directing the epithelial branching, its role is more permissive than instructive [33]. Instead, localized FGF10/FGFR2 signaling in the distal lung is required to regulate the proximal-distal progenitor specification of the developing airway epithelia [34, 33]. The FGF10/FGFR2 signaling activates β-catenin signaling in the proliferating distal tip cells to suppress the expression of SOX2 and induce the expression of SOX9 and ID2. As this SOX9/ID2+ progenitor population expands, their daughter cells get displaced proximally and further away from the FGF10 signaling center concomitant with derepression of SOX2 and subsequent differentiation into bronchial epithelium. Towards the end of the pseudoglandular stage (E15.5), the second wave of FGF10+ cells is dispersed throughout the mesenchyme rather than localized to the distal tip [32]. This dispersion coincides with distal tip progenitors downregulating SOX9 and restricting to the alveolar fate [35]. Overexpression of FGF10, a hyperactive KRAS allele, or SOX9 overexpression from E15.5 blocks the alveolar epithelial differentiation program, maintains the branching program, and induces ectopic expression of proximal lineages [3, 35, 33]. Fascinating recent work has revealed a role for FGF signaling in alveolar epithelial differentiation regulated by fetal breathing movements. Distal progenitors are programmed by the fetal breathing movements to differentiate into the AT1 lineage, but many tip cells have sustained FGF signaling to prevent flattening and retain an AT2-like rather than AT1 cell fate [36].
HIPPO-YAP regulates epithelial progenitor specification and epithelial morphogenesis
The core HIPPO pathway is an evolutionarily conserved serine/threonine kinase signaling cascade composed of MST1/2 kinases, which together with SAV1 and MOB1A/B activate the LATS1/2 kinases to promote phosphorylation of transcriptional coactivators known as YAP and TAZ. In the absence of phosphorylation, YAP and TAZ translocate into the nucleus and regulate expression of genes involved in mechanotransduction, cell proliferation, cell fate specification, and cell death [37]. Deletion of YAP using a variety of pan- and distal epithelial Cre drivers results in a variety of branching morphogenesis and structural defects [38, 39]. Deficiency of TAZ, on the other hand, produces a milder phenotype of abnormal alveolarization mimicking emphysema, suggesting partial overlapping and independent roles of YAP and TAZ [40].
HIPPO pathway’s role in specification of progenitors during lung development came to light when epithelial specific conditional deletion of MST1 (STK4) and MST2 (STK3) resulted in permanent nuclear retention of YAP and TAZ and impaired alveolar epithelial differentiation and proximal multiciliated and secretory cells, while the branching morphogenesis was largely unaffected [41, 42]. Intriguingly, conditional deletion of LATS1/2 kinases, previously thought to be downstream of the MST1/2 pathway, resulted in a different phenotype, with precocious ectopic induction of AT1 cells accompanied by defective branching morphogenesis [43]. Similarly, deletion of YAP and TAZ in the lung epithelium led to a decrease in AT1s, indicating HIPPO signaling as an essential component for AT1 cell fate. Further evidence showing a lack of correlation between the phosphorylation states of LATS kinases and loss of MST1/2 in the lung suggests that MST1/2 might be exploiting a kinase other than LATS1/2 to modify YAP activity during lung development [43]. CLDN18 has also been shown to be an unexpected regulator of nuclear YAP activity that regulates epithelial progenitor proliferation and progenitor capacity in the lung [44]. The compartment-specific roles of the nuclear localization of YAP in later cytodifferentiation of the airways was identified when nucleocytoplasmic shuttling of YAP was shown to be dispensable mainly for both distal branching morphogenesis and distal differentiation, but crucial for proximal airway differentiation [45]. Importantly, loss of YAP in the proximal airways resulted in the increase of multiciliated cells and a decrease of secretory club cells, while constitutive activation of YAP abrogated airway differentiation [41, 45].
TGF-β superfamily member, BMP, patterns the developing airway
The TGF-β superfamily is composed of 33 polypeptides including multiple TGF-β isoforms, activins, nodal, bone morphogenetic proteins (BMPs), and growth and differentiation factors (GDFs). These ligands form homo- and heterodimers and signal through a variety of type II and type I receptor complexes to generate diverse cellular effects via SMAD effector proteins [46]. Appropriate TGF-β signaling is essential for normal lung development with distinct roles in epithelial and mesenchymal compartments. Deletion or overexpression of ligands, ligand-binding partners, receptors and downstream signaling SMADs result in a diverse array of lung structural phenotypes [47].
BMP signaling’s role has been more refined. It is one of the earliest pathways discovered to regulate dorsal-ventral patterning for esophageal and tracheal identity and respiratory specification and morphogenesis [48–50]. Subsequently, BMP was also found to regulate proximal-distal differentiation of epithelial progenitors within the lung. Temporal-dependent inhibition of BMP-BMPR-SMAD signaling in lung development results in various phenotypes including abrupted airway branching morphogenesis and severe reduction in distal epithelial cell types concurrent with an increase in proximal cell types [51–53].
NOTCH signaling is predominantly active in proximal airway progenitor specification
NOTCH signaling is a tightly controlled pathway that regulates precise cell-cell communication during development to regulate survival, proliferation, differentiation and pattering of multiple cell types via cell autonomous as well as cell non-autonomous mechanisms. NOTCH signaling pathway genes are expressed in the developing lung as early as bud formation. Expression of NOTCH1, JAG1 AND JAG2 are restricted to the distal end of the growing bud, while DLL1 expression is found in the proximal region [54].
NOTCH signaling is a critical regulator of progenitor cell specification in early lung development, working predominantly to establish proximal cell lineages. Early explant experiments using NOTCH receptor inhibitors resulted in expansion of distal progenitors, suggesting an importance in proximal-distal patterning [55]. However, subsequent experiments with genetic pan-epithelial deletion of NOTCH components such as POFUT, RBPJK, and JAG1 illustrated a preponderance in directing proximal cell fate [56, 7, 57]. Particlarly, there was a drastic loss of secretory cells and a significant increase in multiciliated and neuroendocrine cells, establishing an important role in proximal cell fate. In addition, the NOTCH target gene, Hes1, was downreguated, corroborating other genetic studies detailing a HES1-ASCL1-IMSL1 transcriptional regulatory network regulating early non-neuroendocrine versus neuroendocrine fate [58, 12, 59]. NOTCH signaling also demonstrates a ligand-dependent role in progenitor cell fate. While NOTCH2 predominantly drives secretory versus multiciliated cell fate, NOTCH1/2/3 play a combinatorial role in specification of neuroendocrine cells [60]. Conversely, misexpression of the NOTCH1 intracellular domain in early lung epithelium expanded secretory cells distally in the lung and decreased multiciliated and increased goblet cells in the trachea [61]. NOTCH3 has also been implicated the balance between TRP63+ basal progenitor and luminal progenitors in a JAG1/JAG2-dependent manner, and it also potentially serves to activate NOTCH1 and NOTCH2 later for secretory and multiciliated cell fate selection in pseudostratified epithelium [62].
Resident epithelial progenitors in the adult lung
The homeostasis and regeneration of the adult lung epithelium is maintained by a variety of cells (Table 2). Homeostasis is an active process with cells required to carry out basic functions of respiration, barrier function, metabolism, and immunity. The cells of the lung have evolved to not only carry out these daily cellular tasks but also simultaneously possess the potential to regenerate tissue following severe injury. Frequently fetal programs are rederived to allow a resident cell to proliferate and differentiate to replace cells at homeostasis and after injury. The terms facultative progenitor, progenitor, and stem cell have all been used to described resident cells of the adult lung that maintain the normal cellular turnover and regenerate the lung following injury. There is ongoing debate on how to accurately classify these cells, and we refer the reader to a recent review on their description in the lung [63]. For simplicity, we will use the term progenitor cell to describe this group of cells.
Table 2.
Adult progenitor cells and progeny at homeostasis and after injury.
| Location | Progenitor cell type | Context | Progeny cell type |
|---|---|---|---|
| Submucosal glands of airway | Myoepithelial cell | Naphthalene, sulfur dioxide, influenza injury | Myoepithelial, transitional basal cell to secretory and multiciliated cells |
| Conducting airway | Basal Cells | Homeostasis | Basal, secretory, tuft, neuroendocrine, and ionocytes |
| Sulfur dioxide, naphthalene | Basal, secretory, multiciliated cells | ||
| Influenza | SOX2+/KRT5+/TRP63+ basal transition cell into AT2s upon inhibition of NOTCH, prevention of hypoxia-induced events, and WNT activation | ||
| Secretory Cells | Homeostasis | Secretory, multiciliated cells | |
| Basal cell depletion, sulfur dioxide, influenza | Dedifferentiated basal, secretory, multiciliated cells | ||
| Naphthalene, sulfur dioxide | Basal, secretory, tuft, neuroendocrine, and ionocytes | ||
| Variant Secretory (NEB niche) | Homeostasis or naphthalene injury | Secretory, multiciliated cells | |
| Bleomycin | AT2s, AT1s | ||
| Bronchioalveolar stem cells | Homeostasis | Secretory and AT2, (rare AT1) | |
| Naphthalene | Secretory, multiciliated cells | ||
| Bleomycin | AT2, AT1 | ||
| Influenza | Secretory, AT2 | ||
| Alveolar | Type 1 | Homeostasis, | AT1 |
| Pneumonectomy | AT1, AT2 | ||
| Type 2 | Homeostasis, bleomycin, AT2 depletion model, pneumonectomy | AT2, AT1 | |
| AEP | Homeostasis | AT2 (rare AT1) | |
| Influenza, lung epithelial deletion model | AT2, AT1 | ||
| a4b6/SFTPC- | Bleomycin | AT2s |
Proximal airway progenitor cells
Submucosal glands
Recent evidence has confirmed the existence of progenitor cells within the submucosal glands of the trachea and lung. The submucosal glands, consisting of ducts and acini, lie within the stromal tissue of the cartilaginous airways throughout the conducting airway in humans, but only in the very proximal portion of the trachea in mice [64]. The glands are lined with epithelial cells including basal, secretory, and multiciliated cells, in addition to a unique myoepithelial cell. Recent studies have implicated this novel myoepithelial progenitor cell in tissue regeneration. Single cell RNA sequencing and cell lineage analysis of ACTA2+ myoepithelial cells within the submucosal glands revealed that these cells obtain a basal cell-like transition with the ability to differentiate into secretory and multiciliated cells [65, 66]. However, this state was only seen in the presence of severe injury, with little to no role in normal homeostasis or mild injury.
Basal cells
BCs are marked by expression of TRP63 and KRT5 and are juxtaposed near the basement membrane of the epithelial layer overlying the cartilaginous airways of the trachea and mainstem bronchi during homeostasis in mice but can extend to the smaller bronchioles in humans [67, 68]. Developmentally, the cells are lineage restricted to their native adult progenitor state and location during branching morphogenesis and can give rise to both secretory and multiciliated cells [69]. At homeostasis in vivo, BCs contribute to the normal cellular turnover of the basal, secretory, and multiciliated cell lineages [68, 70]. One mechanism of maintenance is dependent on mesenchymal-epithelial cross-talk mediated by mesenchymally-derived FGF10 and WNT7B and YAP in the epithelium [71, 33, 72, 73].
Recent studies have expanded BC contribution to additional and novel cell lineages. Single cell RNA sequencing on airway epithelium identified a rare cell type called an ionocyte that is marked by high expression of cystic fibrosis transmembrane conductance regulator (Cftr) gene, indicating a novel cell type for regulation of fluid and ions within the airways [6, 5]. Lineage tracing KRT5+ cells confirmed its presence as a descendent of the basal cell lineage. Similarly, they identified tuft cells and solitary neuroendocrine cells as progeny of basal cells.
Our understanding of basal cell progenitor cell specification derives largely in part from studies of lung regeneration where they aid in re-establishing the airway architecture. In acute injury, BCs proliferate and self renew in addition to differentiating into secretory and multiciliated lineages [68, 74]. Whereas proliferation is NOTCH-independent, differentiation is NOTCH-dependent with promotion of a secretory cell state [75, 76, 62]. Interestingly, maintenence of the secretory state in the daughter cells appears to be dependent on the presence of a persistent NOTCH forward signaling from the parent BC progenitor [77]. Furthermore, the regenerative process may involve subsets of BCs, suggesting a heterogenous BC population with variant potentials for regeneration [75, 70].
Heterogeneity has also been demonstrated in influenza models of lung injury in mice. KRT5+/TRP63+ cells arise in distinct clusters of epitheloid pods marking areas of severe lung damage after influenza injury in mice [78]. Further characterization using fate mapping revealed that they likely arise from subsets of immature basal-like cells present in the airways [79, 80, 69, 81]. While initially proposed to be a significant component of alveolar epithelial cell regeneration, KRT5+/TRP63+ pods’ main function is to cover damaged and exposed basement membrane to maintain integrity rather than replacement of alveolar epithelial cells [80, 82]. However, these areas of severe injury are marked by excessive NOTCH signaling and local hypoxia with upregulation of HIF1A. Inhibition of NOTCH signaling, deletion of HIF1A, or β-catenin activation all promote alveolar epithelial cell differentiation from KRT5+/TRP63+ pods, revealing a potential therapeutic target in regeneration of severely damaged tissue [80, 83].
Secretory Cells
Secretory or club cells are found throughout the trachea and conducting airway in mice, but predominantly in only intrapulmonary airways in humans [84]. At homeostasis and during repair, secretory cells can self-renew and differentiate into multiciliated cells [84]. In addition, in models of basal cell depletion, they replicate and dedifferentiate into BCs to replace a portion of this population [71]. Like BCs, the secretory cell population is likely heterogenous with divergent responses in homeostasis and injury [6, 5, 10]. For example, variant club cells form a niche surrounding the pulmonary neuroendocrine bodies in the proximal airways in mice [85]. The variant club cell population is marked by expression of UPK3A, and fate mapping UPK3A+ cells revealed they act as progenitors for secretory and multiciliated cells in adult homeostasis and regeneration following naphthalene injury [86].
Bronchioalveolar stem cells (BASCs)
The presence of BASCs was first suggested in a study investigating a potential stem cell population at the terminal bronchioles following naphthalene injury [87]. These cells were resistant to naphthalene, expressed SCGB1A1, and proliferated after injury. Additional evidence using dual immunofluorescence for the AT2 marker, SFTPC, and SCGB1A1 noted the presence of this potential dual lineage at the bronchoalveolar ductal junction in normal lung and in lung tumors [88]. More recently, definitive evidence was presented using two novel dual lineage labeling strategies that allow for tracking cells only expressing both Sftpc and Scgb1a1 [89, 90]. By using a split-intein-mediated effector reconstitution system or the Cre/Dre recombinase dual recombination system, the two groups were able to label the rare BASCs at the bronchoalveolar ductal junction. Upon different injuries specific for airway, alveolus, or both, they revealed expansion of the BASC population in tissue repair. Segregating this cell population and performing RNA sequencing revealed a BASC gene signature that shared the transcriptomic repertoire of both AT2s and secretory cells. Interestingly, SCA1, a putative marker for BASCs in the lung, was expressed rarely on BASCs by flow cytometry, indicating that it may not be a sufficient marker for all BASCs. Interestingly, there was low expression of the naphthalene metabolizing enzyme, Cyp2f2, which may explain their resistance to naphthalene injury.
Distal airway progenitor cells
Alveolar epithelial cells
The adult alveolus is comprised of two types of epithelium. AT1s are elongated, flat cells that cover the alveolar surface and exchange oxygen with the capillary network. The AT2s are a cuboidal, highly metabolic cell with multiple functions including the production of surfactant. Historically, descriptive studies suggested that AT2s could differentiate into AT1s in the presence of injury [91]. More recently, confirmatory experiments using fate mapping of AT2 revealed that AT2s underwent self-renewal with rare AT1 differentiation during homeostasis [92]. AT1 lineage commitment from AT2s was significantly increased following bleomycin-induced alveolar injury or AT2 depletion [92, 93]. Conversely, the AT1 plasticity has also been illustrated in a pneumonectomy model of alveolar growth in that lineage labeled AT1s exhibit the ability to differentiate into AT2s [94]. While mechanisms of plasticity in AT1s is largely unclear, AT2 progenitor cell proliferation and differentiation following injury is in part regulated by a number of signaling pathways including the BMP, NOTCH, and HIPPO signaling [95–97].
Data also indicate the presence of several novel alveolar epithelial lineages. A population of SFTPC-, α6β4+ epithelial cells reside in the alveolus and bronchoalveolar ductal junction [98]. Following injury or in organoid modeling systems, the cells appear to proliferate and differentiate into AT2s. However, these data were generated using novel ex vivo systems rather than classical fate mapping of this specific lineage. As such, confirmatory studies using recent dual lineage labeling strategies would make it possible to track this potential progenitor cell in vivo.
More recently, subsets of alveolar epithelial progenitor cells were identified based on single cell and population-based RNA sequencing and their ability to respond to a WNT signal [99, 100]. This WNT-responsive alveolar epithelial lineage arises as a subset of AT2s (AT2Axin2) during alveologenesis and orchestrates the AT2 pool through enhanced proliferation and inhibition of AT1 differentiation [27]. During alveologenesis, it is a dynamic population with some AT2s gaining or losing WNT-responsiveness. However, in the adult, it becomes a small, stable alveolar epithelial progenitor (AEP) population that is poised for regeneration based on transcriptome enrichment and chromatin architecture. After influenza injury, AEPs preferentially proliferate to replace AT2s and later differentiate to contribute to some AT1 regeneration [99, 100]. While AEPs appear to contribute significantly to AT2 regeneration surrounding areas of moderate injury following influenza infection, their contribution in other injury models is unclear.
Conclusion
The lung is not a quiescent organ and requires an orchestra of cellular components to interact and carry out the basic functions of respiration. To achieve this complexity, progenitor cells must receive and integrate signals from their respective niches. We have outlined some of the major fundamental morphogen signaling systems involved in lung development to provide a foundation for understanding progenitor cell specification and maintenance. These pathways provide a roadmap for progenitor cell specification in lung regeneration. Technology continues to advance discoveries in biology and uncover novel signaling pathways conducting progenitor cell fate. Recent insights in the lung biology field have taken advantage of these tools to identify new lineages. Lineages that now will entice investigations to understand their ontogeny, morphogenesis, and contribution to lung homeostasis and regeneration. While most lung disease today has no cure, model organisms provide a blueprint for therapy. Whether we can genuinely replicate human disease is not known. However, our advances in imaging, single cell analysis, and computational trajectory mapping of lineages will help allieviate the constraints in human disease samples. At the same time, we must bridge knowledge unraveling regulatory mechanisms in fate decisions of other organ systems with prospective discovery in lung biology. It is only with integration of knowledge and novel tools we will be able to direct therapy in lung regeneration.
Acknowledgements
Due to space limitations, we apologize to our scientific colleagues whose work could not be cited. We would like to thank Dr. Jarod Zepp for critical review of this manuscript. This work was supported by grants from the National Institutes of Health K08-HL140129 (D.B.F), the Parker B. Francis Foundation (D.B.F.), and the W.W. Smith Charitable Trust.
Footnotes
Conflict of Interest
Aravind Sivakumar and David B. Frank declare that they have no conflict of interest
Human and Animal Rights and Informed Consent
This manuscript does not contain any original studies involving human or animal subjects.
References
Papers of particular interest and published recently have been highlighted as:
• Of importance
•• Of major importance
- 1.Franks TJ, Colby TV, Travis WD, Tuder RM, Reynolds HY, Brody AR et al. Resident cellular components of the human lung: current knowledge and goals for research on cell phenotyping and function. Proc Am Thorac Soc. 2008;5(7):763–6. doi: 10.1513/pats.200803-025HR. [DOI] [PubMed] [Google Scholar]
- 2.Minoo P, Su G, Drum H, Bringas P, Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(−/−) mouse embryos. Dev Biol. 1999;209(1):60–71. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- 3.Alanis DM, Chang DR, Akiyama H, Krasnow MA, Chen J. Two nested developmental waves demarcate a compartment boundary in the mouse lung. Nat Commun. 2014;5:3923. doi: 10.1038/ncomms4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136(11):1899–907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plasschaert LW, Zilionis R, Choo-Wing R, Savova V, Knehr J, Roma G et al. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature. 2018;560(7718):377–81. doi: 10.1038/s41586-018-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identifies a new recently discovered cell type called pulmonary ionocyte in the conducting airways which is a major source of CFTR (gene mutated in cystic fibrosis) activity in the lungs being regulated by Notch and FoxI1 signaling.
- 6.Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 2018;560(7718):319–24. doi: 10.1038/s41586-018-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Defines the place of the ionocyte in the epithelial hierarchy by proving that the newly identified ionocyte cell type is a part of high turnover set of epithelial cells that are continuously replenished by basal progenitor cells regulated by FoxI1.
- 7.Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development. 2009;136(13):2297–307. doi: 10.1242/dev.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morimoto M, Liu Z, Cheng HT, Winters N, Bader D, Kopan R. Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J Cell Sci. 2010;123(Pt 2):213–24. doi: 10.1242/jcs.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rawlins EL, Ostrowski LE, Randell SH, Hogan BL. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci U S A. 2007;104(2):410–7. doi: 10.1073/pnas.0610770104. [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes elegant lineage tracing experiments using the Id2 cre driver which established the progressive specification and differentiation of the distal tip of the airways.
- 10.Reynolds SD, Reynolds PR, Pryhuber GS, Finder JD, Stripp BR. Secretoglobins SCGB3A1 and SCGB3A2 define secretory cell subsets in mouse and human airways. Am J Respir Crit Care Med. 2002;166(11):1498–509. doi: 10.1164/rccm.200204-285OC. [DOI] [PubMed] [Google Scholar]
- 11.Post LC, Ternet M, Hogan BL. Notch/Delta expression in the developing mouse lung. Mech Dev. 2000;98(1–2):95–8. [DOI] [PubMed] [Google Scholar]
- 12.Ito T, Udaka N, Yazawa T, Okudela K, Hayashi H, Sudo T et al. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development. 2000;127(18):3913–21. [DOI] [PubMed] [Google Scholar]
- 13.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci U S A. 2002;99(16):10482–7. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009;136(22):3741–5. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank DB, Penkala IJ, Zepp JA, Sivakumar A, Linares-Saldana R, Zacharias WJ et al. Early lineage specification defines alveolar epithelial ontogeny in the murine lung. Proc Natl Acad Sci U S A. 2019. doi: 10.1073/pnas.1813952116. [DOI] [PMC free article] [PubMed] [Google Scholar]; Suggests that alveolar specification at the distal tips initiated concurrent with the branching morphogenesis during the pseudoglandular stage changing the timeline of alveolar specification.
- 16.Treutlein B, Brownfield DG, Wu AR, Neff NF, Mantalas GL, Espinoza FH et al. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature. 2014;509(7500):371–5. doi: 10.1038/nature13173. [DOI] [PMC free article] [PubMed] [Google Scholar]; First study to use single cell RNA sequencing to describe the lineage hierarchies to support the progressive specification and differentiation model of alveologenesis.
- 17. •.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507(7491):190–4. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]; Proposes the existence of a novel bipotent progenitor population at the distal tip of the airways that gave rise to the alveolar type I and type II cells during alveologenesis and its relevance in cancer.
- 18.Steinhart Z, Angers S. Wnt signaling in development and tissue homeostasis. Development. 2018;145(11). doi: 10.1242/dev.146589. [DOI] [PubMed] [Google Scholar]
- 19.De Langhe SP, Reynolds SD. Wnt signaling in lung organogenesis. Organogenesis. 2008;4(2):100–8. doi: 10.4161/org.4.2.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM et al. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17(2):290–8. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostrin EJ, Little DR, Gerner-Mauro KN, Sumner EA, Ríos-Corzo R, Ambrosio E et al. β-Catenin maintains lung epithelial progenitors after lung specification. Development. 2018;145(5):dev160788. doi: 10.1242/dev.160788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris-Johnson KS, Domyan ET, Vezina CM, Sun X. beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc Natl Acad Sci U S A. 2009;106(38):16287–92. doi: 10.1073/pnas.0902274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Langhe SP, Sala FG, Del Moral P-M, Fairbanks TJ, Yamada KM, Warburton D et al. Dickkopf-1 (DKK1) reveals that fibronectin is a major target of Wnt signaling in branching morphogenesis of the mouse embryonic lung. Developmental Biology. 2005;277(2):316–31. doi: 10.1016/j.ydbio.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 24.Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM et al. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol. 2005;283(1):226–39. doi: 10.1016/j.ydbio.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto S, Chen H, Que J, Brockway BL, Drake JA, Snyder JC et al. beta-Catenin-SOX2 signaling regulates the fate of developing airway epithelium. J Cell Sci. 2012;125(Pt 4):932–42. doi: 10.1242/jcs.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okubo T, Hogan BL. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol. 2004;3(3):11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frank DB, Peng T, Zepp JA, Snitow M, Vincent TL, Penkala IJ et al. Emergence of a Wave of Wnt Signaling that Regulates Lung Alveologenesis by Controlling Epithelial Self-Renewal and Differentiation. Cell Reports. 2016;17(9):2312–25. doi: 10.1016/j.celrep.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ornitz DM, Itoh N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4(3):215–66. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. 2005;132(1):35–47. doi: 10.1242/dev.01570. [DOI] [PubMed] [Google Scholar]
- 30.Yuan T, Volckaert T, Chanda D, Thannickal VJ, De Langhe SP. Fgf10 Signaling in Lung Development, Homeostasis, Disease, and Repair After Injury. Front Genet. 2018;9:418. doi: 10.3389/fgene.2018.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T et al. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21(1):138–41. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- 32.El Agha E, Herold S, Al Alam D, Quantius J, MacKenzie B, Carraro G et al. Fgf10-positive cells represent a progenitor cell population during lung development and postnatally. Development. 2014;141(2):296–306. doi: 10.1242/dev.099747. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows the existence of two temporal waves of FGF signaling and its contribution to lung progenitor specification and development.
- 33.Volckaert T, Campbell A, Dill E, Li C, Minoo P, De Langhe S. Localized Fgf10 expression is not required for lung branching morphogenesis but prevents differentiation of epithelial progenitors. Development. 2013;140(18):3731. doi: 10.1242/dev.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abler LL, Mansour SL, Sun X. Conditional gene inactivation reveals roles for Fgf10 and Fgfr2 in establishing a normal pattern of epithelial branching in the mouse lung. Dev Dyn. 2009;238(8):1999–2013. doi: 10.1002/dvdy.22032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang DR, Martinez Alanis D, Miller RK, Ji H, Akiyama H, McCrea PD et al. Lung epithelial branching program antagonizes alveolar differentiation. Proc Natl Acad Sci U S A. 2013;110(45):18042–51. doi: 10.1073/pnas.1311760110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Wang Z, Chu Q, Jiang K, Li J, Tang N. The Strength of Mechanical Forces Determines the Differentiation of Alveolar Epithelial Cells. Developmental Cell. 2018;44(3):297–312.e5. doi: 10.1016/j.devcel.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Misra JR, Irvine KD. The Hippo Signaling Network and Its Biological Functions. Annual Review of Genetics. 2018;52(1):65–87. doi: 10.1146/annurev-genet-120417-031621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin C, Yao E, Zhang K, Jiang X, Croll S, Thompson-Peer K et al. YAP is essential for mechanical force production and epithelial cell proliferation during lung branching morphogenesis. eLife. 2017;6:e21130. doi: 10.7554/eLife.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahoney John E, Mori M, Szymaniak Aleksander D, Varelas X, Cardoso Wellington V. The Hippo Pathway Effector Yap Controls Patterning and Differentiation of Airway Epithelial Progenitors. Developmental Cell. 2014;30(2):137–50. doi: 10.1016/j.devcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makita R, Uchijima Y, Nishiyama K, Amano T, Chen Q, Takeuchi T et al. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. American Journal of Physiology-Renal Physiology. 2008;294(3):F542–F53. doi: 10.1152/ajprenal.00201.2007. [DOI] [PubMed] [Google Scholar]
- 41.Lange AW, Sridharan A, Xu Y, Stripp BR, Perl A-K, Whitsett JA. Hippo/Yap signaling controls epithelial progenitor cell proliferation and differentiation in the embryonic and adult lung. Journal of Molecular Cell Biology. 2014;7(1):35–47. doi: 10.1093/jmcb/mju046 %J Journal of Molecular Cell Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin C, Yao E, Chuang PT. A conserved MST1/2-YAP axis mediates Hippo signaling during lung growth. Dev Biol. 2015;403(1):101–13. doi: 10.1016/j.ydbio.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nantie LB, Young RE, Paltzer WG, Zhang Y, Johnson RL, Verheyden JM et al. <em>Lats1/2 inactivation reveals Hippo function in alveolar type I cell differentiation during lung transition to air breathing. Development. 2018;145(21):dev163105. doi: 10.1242/dev.163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou B, Flodby P, Luo J, Castillo DR, Liu Y, Yu F-X et al. Claudin-18–mediated YAP activity regulates lung stem and progenitor cell homeostasis and tumorigenesis. The Journal of Clinical Investigation. 2018;128(3):970–84. doi: 10.1172/JCI90429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. •.van Soldt BJ, Qian J, Li J, Tang N, Lu J, Cardoso WV. Yap and its subcellular localization have distinct compartment-specific roles in the developing lung. Development. 2019:dev.175810. doi: 10.1242/dev.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]; Consists of elegantly designed experiments that were able to resolve the controversies surrounding the complex role of HIPPO-YAP pathway in lung specification.
- 46.Weiss A, Attisano L. The TGFbeta Superfamily Signaling Pathway. Wiley Interdisciplinary Reviews: Developmental Biology. 2013;2(1):47–63. doi: 10.1002/wdev.86. [DOI] [PubMed] [Google Scholar]
- 47.Aschner Y, Downey GP. Transforming Growth Factor-beta: Master Regulator of the Respiratory System in Health and Disease. Am J Respir Cell Mol Biol. 2016;54(5):647–55. doi: 10.1165/rcmb.2015-0391TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Domyan ET, Ferretti E, Throckmorton K, Mishina Y, Nicolis SK, Sun X. Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development. 2011;138(5):971–81. doi: 10.1242/dev.053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Gordon J, Manley NR, Litingtung Y, Chiang C. Bmp4 is required for tracheal formation: a novel mouse model for tracheal agenesis. Dev Biol. 2008;322(1):145–55. doi: 10.1016/j.ydbio.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation. 2006;74(7):422–37. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 51.Weaver M, Yingling JM, Dunn NR, Bellusci S, Hogan BL. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development. 1999;126(18):4005. [DOI] [PubMed] [Google Scholar]
- 52.Sun J, Chen H, Chen C, Whitsett JA, Mishina Y, Bringas P Jr. et al. Prenatal Lung Epithelial Cell-Specific Abrogation of Alk3-Bone Morphogenetic Protein Signaling Causes Neonatal Respiratory Distress by Disrupting Distal Airway Formation. The American Journal of Pathology. 2008;172(3):571–82. doi: 10.2353/ajpath.2008.070286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eblaghie MC, Reedy M, Oliver T, Mishina Y, Hogan BL. Evidence that autocrine signaling through Bmpr1a regulates the proliferation, survival and morphogenetic behavior of distal lung epithelial cells. Dev Biol. 2006;291(1):67–82. doi: 10.1016/j.ydbio.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 54.Kong Y, Glickman J, Subramaniam M, Shahsafaei A, Allamneni KP, Aster JC et al. Functional diversity of notch family genes in fetal lung development. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2004;286(5):L1075–L83. doi: 10.1152/ajplung.00438.2002. [DOI] [PubMed] [Google Scholar]
- 55.Tsao PN, Chen F, Izvolsky KI, Walker J, Kukuruzinska MA, Lu J et al. Gamma-secretase activation of notch signaling regulates the balance of proximal and distal fates in progenitor cells of the developing lung. J Biol Chem. 2008;283(43):29532–44. doi: 10.1074/jbc.M801565200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsao P-N, Wei S-C, Wu M-F, Huang M-T, Lin H-Y, Lee M-C et al. Notch signaling prevents mucous metaplasia in mouse conducting airways during postnatal development. Development. 2011;138(16):3533. doi: 10.1242/dev.063727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang S, Loch AJ, Radtke F, Egan SE, Xu K. Jagged1 is the major regulator of notch-dependent cell fate in proximal airways. Developmental Dynamics. 2013;242(6):678–86. doi: 10.1002/dvdy.23965. [DOI] [PubMed] [Google Scholar]
- 58.Borges M, Linnoila RI, van de Velde HJ, Chen H, Nelkin BD, Mabry M et al. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature. 1997;386(6627):852–5. doi: 10.1038/386852a0. [DOI] [PubMed] [Google Scholar]
- 59.Jia S, Wildner H, Birchmeier C. Insm1 controls the differentiation of pulmonary neuroendocrine cells by repressing Hes1. Dev Biol. 2015;408(1):90–8. doi: 10.1016/j.ydbio.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 60.Morimoto M, Nishinakamura R, Saga Y, Kopan R. Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development. 2012;139(23):4365–73. doi: 10.1242/dev.083840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA et al. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136(10):1751. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mori M, Mahoney JE, Stupnikov MR, Paez-Cortez JR, Szymaniak AD, Varelas X et al. Notch3-Jagged signaling controls the pool of undifferentiated airway progenitors. Development. 2015;142(2):258. doi: 10.1242/dev.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leach JP, Morrisey EE. Repairing the lungs one breath at a time: How dedicated or facultative are you? Genes Dev. 2018;32(23–24):1461–71. doi: 10.1101/gad.319418.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lynch TJ, Engelhardt JF. Progenitor cells in proximal airway epithelial development and regeneration. J Cell Biochem. 2014;115(10):1637–45. doi: 10.1002/jcb.24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tata A, Kobayashi Y, Chow RD, Tran J, Desai A, Massri AJ et al. Myoepithelial Cells of Submucosal Glands Can Function as Reserve Stem Cells to Regenerate Airways after Injury. Cell Stem Cell. 2018;22(5):668–83 e6. doi: 10.1016/j.stem.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; Uncovers the submucosal myoepithelial cell as a progenitor cell in airway regeneration.
- 66.Lynch TJ, Anderson PJ, Rotti PG, Tyler SR, Crooke AK, Choi SH et al. Submucosal Gland Myoepithelial Cells Are Reserve Stem Cells That Can Regenerate Mouse Tracheal Epithelium. Cell Stem Cell. 2018;22(5):653–67 e5. doi: 10.1016/j.stem.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]; Uncovers the submucosal myoepithelial cell as a progenitor cell in airway regeneration.
- 67.Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am J Respir Crit Care Med. 1998;157(6 Pt 1):2000–6. doi: 10.1164/ajrccm.157.6.9707011. [DOI] [PubMed] [Google Scholar]
- 68.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106(31):12771–5. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang Y, Riccio P, Schotsaert M, Mori M, Lu J, Lee DK et al. Spatial-Temporal Lineage Restrictions of Embryonic p63(+) Progenitors Establish Distinct Stem Cell Pools in Adult Airways. Dev Cell. 2018;44(6):752–61 e4. doi: 10.1016/j.devcel.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watson JK, Rulands S, Wilkinson AC, Wuidart A, Ousset M, Van Keymeulen A et al. Clonal Dynamics Reveal Two Distinct Populations of Basal Cells in Slow-Turnover Airway Epithelium. Cell Rep. 2015;12(1):90–101. doi: 10.1016/j.celrep.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503(7475):218–23. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao R, Fallon TR, Saladi SV, Pardo-Saganta A, Villoria J, Mou H et al. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev Cell. 2014;30(2):151–65. doi: 10.1016/j.devcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balasooriya GI, Goschorska M, Piddini E, Rawlins EL. FGFR2 is required for airway basal cell self-renewal and terminal differentiation. Development. 2017;144(9):1600. doi: 10.1242/dev.135681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghosh M, Brechbuhl HM, Smith RW, Li B, Hicks DA, Titchner T et al. Context-dependent differentiation of multipotential keratin 14-expressing tracheal basal cells. Am J Respir Cell Mol Biol. 2011;45(2):403–10. doi: 10.1165/rcmb.2010-0283OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pardo-Saganta A, Law BM, Tata PR, Villoria J, Saez B, Mou H et al. Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell. 2015;16(2):184–97. doi: 10.1016/j.stem.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8(6):639–48. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identifies NOTCH mediated mechanisms that regulate differentiation of basal cells towards the secretory lienages during repair post lung injury.
- 77.Pardo-Saganta A, Tata PR, Law BM, Saez B, Chow RD-W, Prabhu M et al. Parent stem cells can serve as niches for their daughter cells. Nature. 2015;523:597. doi: 10.1038/nature14553. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reveals that airway basal stem/progenitor cells continuously supply a NOTCH ligand to their daughter secretory cells which was critical in maintaining the progenitor pool.
- 78.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147(3):525–38. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ray S, Chiba N, Yao C, Guan X, McConnell AM, Brockway B et al. Rare SOX2(+) Airway Progenitor Cells Generate KRT5(+) Cells that Repopulate Damaged Alveolar Parenchyma following Influenza Virus Infection. Stem Cell Reports. 2016;7(5):817–25. doi: 10.1016/j.stemcr.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517(7536):621–5. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]; Characterizes a rare lineage-negative epithelial stem/progenitor (LNEP) cells present within normal distal lung which assembled into the KRT5 pods that form during influenza injuries and importantly identified NOTCH mediated pathways that promoted the differentiation of these cells to reconstitute the alveoli.
- 81.Zuo W, Zhang T, Wu DZ, Guan SP, Liew AA, Yamamoto Y et al. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517(7536):616–20. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kanegai CM, Xi Y, Donne ML, Gotts JE, Driver IH, Amidzic G et al. Persistent Pathology in Influenza-Infected Mouse Lungs. Am J Respir Cell Mol Biol. 2016;55(4):613–5. doi: 10.1165/rcmb.2015-0387LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xi Y, Kim T, Brumwell AN, Driver IH, Wei Y, Tan V et al. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nature Cell Biology. 2017;19:904. doi: 10.1038/ncb3580. [DOI] [PMC free article] [PubMed] [Google Scholar]; Uncovers unique pathways critical for converting KRT5+ pods into alveolar epithelium for lung regneration after influenza injury.
- 84.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4(6):525–34. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guha A, Vasconcelos M, Cai Y, Yoneda M, Hinds A, Qian J et al. Neuroepithelial body microenvironment is a niche for a distinct subset of Clara-like precursors in the developing airways. Proc Natl Acad Sci U S A. 2012;109(31):12592–7. doi: 10.1073/pnas.1204710109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guha A, Deshpande A, Jain A, Sebastiani P, Cardoso WV. Uroplakin 3a(+) Cells Are a Distinctive Population of Epithelial Progenitors that Contribute to Airway Maintenance and Post-injury Repair. Cell Rep. 2017;19(2):246–54. doi: 10.1016/j.celrep.2017.03.051. [DOI] [PubMed] [Google Scholar]
- 87.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161(1):173–82. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–35. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 89.Liu Q, Liu K, Cui G, Huang X, Yao S, Guo W et al. Lung regeneration by multipotent stem cells residing at the bronchioalveolar-duct junction. Nat Genet. 2019;51(4):728–38. doi: 10.1038/s41588-019-0346-6. [DOI] [PubMed] [Google Scholar]; Using a novel dual recombinase lineage tracing tool, confirms the existance of BASCs and their importance in lung regeneration after injury.
- 90.Salwig I, Spitznagel B, Vazquez-Armendariz AI, Khalooghi K, Guenther S, Herold S et al. Bronchioalveolar stem cells are a main source for regeneration of distal lung epithelia in vivo. EMBO J. 2019..doi: 10.15252/embj.2019102099. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a novel dual lineage tracing reconstitutive Cre recombinase tool, confirms the existance of BASCs and their importance in lung regeneration after injury
- 91.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Renewal of alveolar epithelium in the rat following exposure to NO2. Am J Pathol. 1973;70(2):175–98. [PMC free article] [PubMed] [Google Scholar]
- 92.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123(7):3025–36. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]; First study to show the self-renewal and the differentiation capabilities of the AT2 cells in the alveoli.
- 93.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108(52):E1475–83. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jain R, Barkauskas CE, Takeda N, Bowie EJ, Aghajanian H, Wang Q et al. Plasticity of Hopx(+) type I alveolar cells to regenerate type II cells in the lung. Nat Commun. 2015;6:6727. doi: 10.1038/ncomms7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chung MI, Bujnis M, Barkauskas CE, Kobayashi Y, Hogan BLM. Niche-mediated BMP/SMAD signaling regulates lung alveolar stem cell proliferation and differentiation. Development. 2018;145(9). doi: 10.1242/dev.163014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.LaCanna R, Liccardo D, Zhang P, Tragesser L, Wang Y, Cao T et al. Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J Clin Invest. 2019;130:2107–22. doi: 10.1172/JCI125014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Finn J, Sottoriva K, Pajcini KV, Kitajewski JK, Chen C, Zhang W et al. Dlk1-Mediated Temporal Regulation of Notch Signaling Is Required for Differentiation of Alveolar Type II to Type I Cells during Repair. Cell Rep. 2019;26(11):2942–54 e5. doi: 10.1016/j.celrep.2019.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K et al. Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121(7):2855–62. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA, Desai TJ. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science. 2018;359(6380):1118–23. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]; Highlights the importance of Wnt signaling in regulating the stemness and transdifferential potential of a subset of AT2 cells and its role in mediating the crosstalk with the neighboring Wnt secreting fibroblasts during homeostasis and injury.
- 100.Zacharias WJ, Frank DB, Zepp JA, Morley MP, Alkhaleel FA, Kong J et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555:251. doi:10.1038/nature2578610.1038/nature25786https://www.nature.com/articles/nature25786#supplementary-informationhttps://www.nature.com/articles/nature25786#supplementary-information . [DOI] [PMC free article] [PubMed] [Google Scholar]; Identifies a stable, Wnt responsive subpopulation of AT2 cells that are poised for regeneration during homeostasis which upon influenza mediated injury, contribute significantly to the regeneration of alveoli.