Fig. 3.
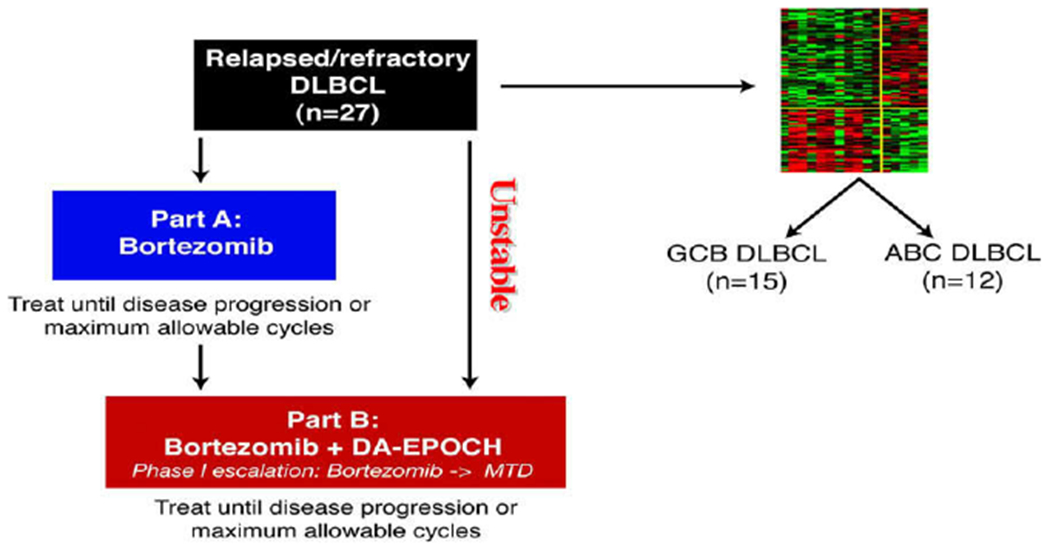
Study schema. Patients initially received bortezomib alone at 1.3 mg/m2 on days 1, 4, 8, and 11 every 21 days (Part A) unless they had disease that the investigators judged to require immediate chemotherapy, as in cases of impending or ongoing organ compromise; these patients received only Part B. Patients with progressive disease in Part A later received bortezomib with DA-EPOCH (Part B). Molecular classification. Of 31 DLBCL cases analyzed by gene expression profiling, 16 were excluded due to ineligible subtype by classification or did not receive Part A, leaving 5 ABC and 10 GCB cases eligible for analysis of outcome. Of 24 paraffin embedded tumor biopsies analyzed by immunohistochemistry, 12 of each were categorized as GCB and ABC type.By combining both methods, cases were identified as GCB in 15 and ABC in 12 and included in the analysis of outcome with Part B.
