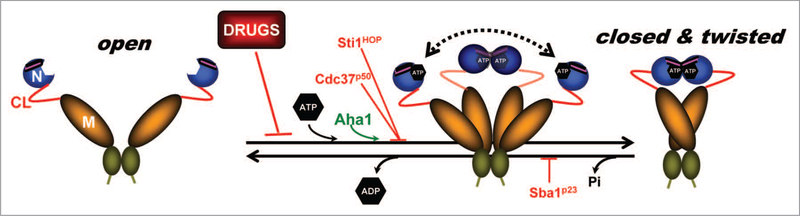
Figure 1.
Model depicting the Hsp90 chaperone cycle. ATP binding to the N-terminal domains of Hsp90 (open) promotes repositioning of a “lid” segment followed by transient dimerization of the N-domains. Subsequent structural rearrangements result in the (closed and twisted) conformation of Hsp90 that is competent for ATP hydrolysis. Binding of the co-chaperone Aha1 enhances Hsp90 ATPase activity. The co-chaperones Sti1/HOP and Cdc37/p50, or pharmacologic inhibitors such as geldanamycin or radicicol, exert an opposite effect by blocking the initial structural changes necessary for N-domain dimerization. Sba1/p23 strengthens the late Hsp90 conformation and inhibits ATP hydrolysis. Domain labeling is as follows: N, N-domain (blue); CL, charged linker (red); M, M-domain (yellow); C, C-domain (green); ATP lid, (purple).