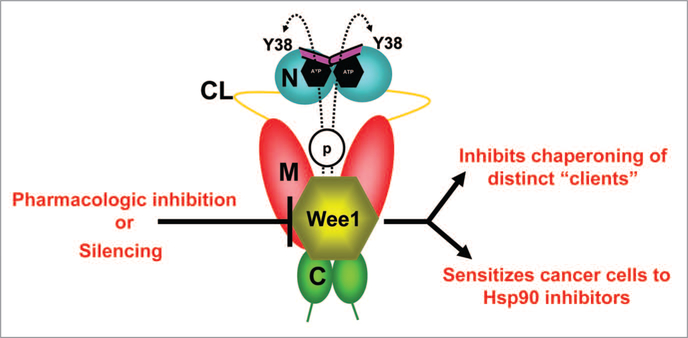
Figure 3.
wee1, an Hsp90 client protein, phosphorylates a conserved tyrosine residue (Y38) in the N-domain of a subpopulation of nuclear-localized yHsp90. Phosphorylation also leads to ubiquitination and degradation of Hsp90 by cytoplasmic proteasomes. Pharmacologic inhibition/molecular silencing of wee1 inhibits Hsp90 chaperoning of distinct clients and sensitizes cells to Hsp90 inhibitor-induced apoptosis. Domain labeling is as follows: N, N-domain (blue); CL, charged linker (yellow); M, M-domain (red); C, C-domain (green); ATP lid, (purple).