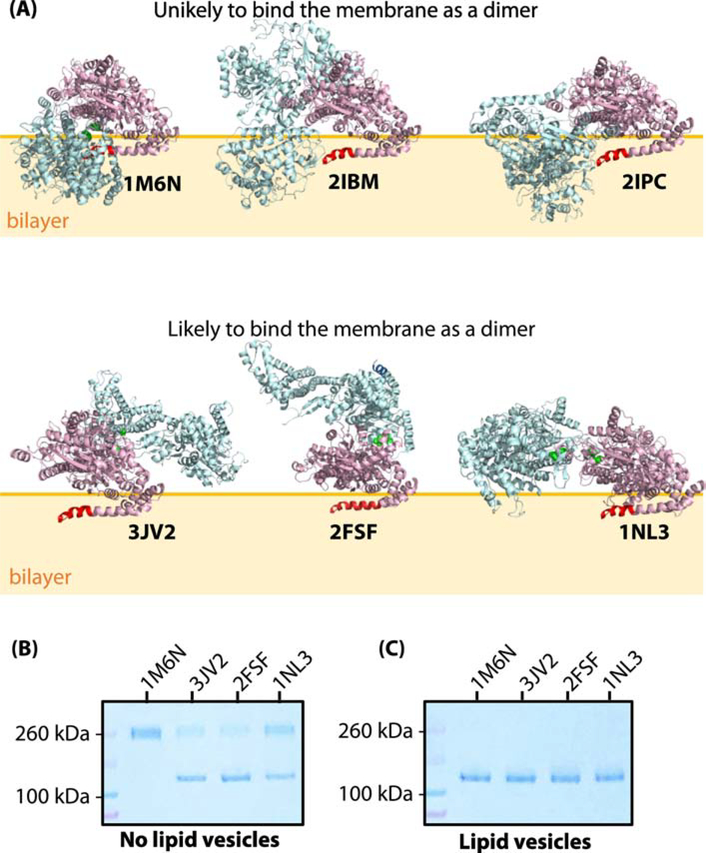
Figure 1.
SecA does not bind as one of the crystallographic dimers to large unilamellar vesicles (LUV) formed from Escherichia coli lipids. (A) Three-dimensional structures of six reported crystallographic dimers. The N-terminus of SecA, which is necessary for membrane binding [10, 50], is highlighted in red in one of the protomers. The top panel corresponds to the dimer species that are unlikely to bind to the membrane without major steric clashes with the membrane. In support of this hypothesis, we note that an earlier study we showed that the 1M6N dimer cannot bind significantly to E. coli LUV [10]. The bottom panel shows the dimer species that potentially could exist on the lipid bilayer without steric clashes with the bilayer. (B) Coomassie-blue stained SDS-PAGE denaturing gel of cysteine-mutants of SecA (1 μM) in the absence of LUVs under oxidizing conditions. Note the presence of both monomers and dimers for 3JV2, 2FSF, and 1NL3 but not 1M6N. (C) Coomassie-blue stained SDS-PAGE gel of cysteine-mutants of SecA (1 μM) in the presence of LUVs (E. coli lipids, 6 mM). Partitioning of SecA was allowed for 30 minutes prior to introducing the oxidizer. In this case, no dimers are observed.